100 Ml Over 90 Minutes Rate
Hospitals nationwide are scrambling to address a critical medication delivery issue: the administration rate of certain intravenous (IV) medications, specifically a 100 ml fluid volume infused over 90 minutes. This seemingly minor adjustment has triggered widespread concern due to potential adverse patient reactions and regulatory scrutiny.
The rapid adoption of electronic health records (EHRs) and automated dispensing systems (ADS) has inadvertently highlighted discrepancies in previously accepted manual practices. The focus is now on ensuring precise medication delivery to minimize risks.
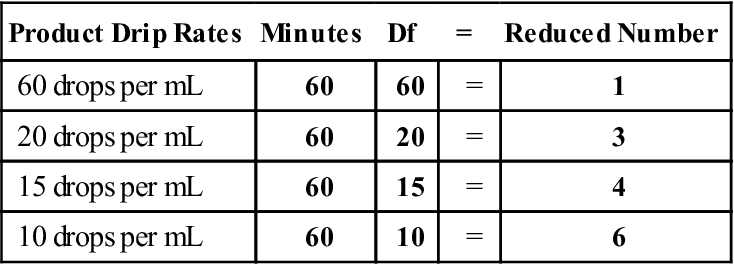
The Core Issue: 100 ml Over 90 Minutes
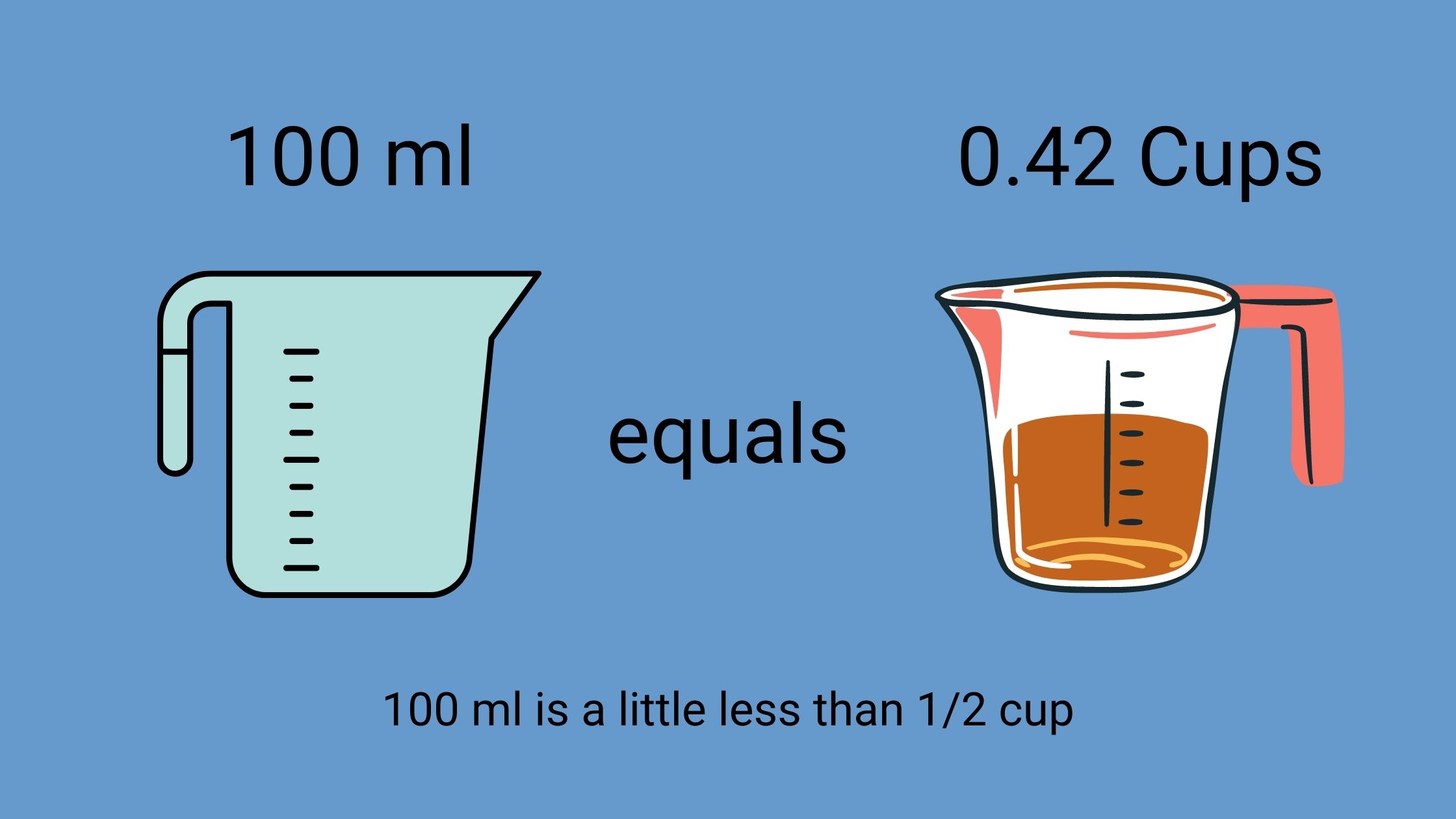
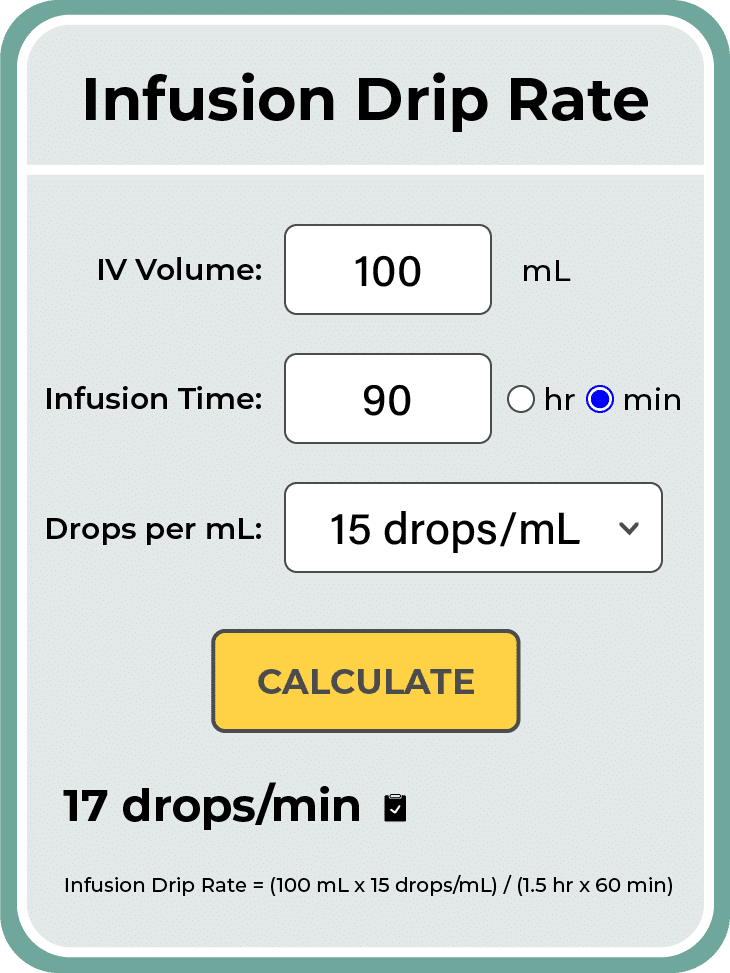
The central problem lies in the recommended infusion rate for specific medications. A 100 ml IV bag, intended to be administered over 90 minutes, has become a focal point of investigation due to increased incident reports.
These reports detail adverse events ranging from mild discomfort to potentially severe complications when the infusion rate deviates from established guidelines. These incidents highlight the vulnerability in medication delivery protocols.
Who is Affected?
Patients receiving specific medications requiring precise administration rates are directly affected. Nurses and other healthcare providers responsible for administering these medications face increased scrutiny and potential liability.
Hospitals and healthcare systems are grappling with the logistical challenges of updating protocols and retraining staff. Pharmaceutical companies that manufacture affected medications are also under pressure to provide clearer guidelines.
What Medications are Involved?
While the specific medications involved are numerous and vary by hospital, common examples include certain antibiotics, antifungals, and chemotherapeutic agents. Medications with narrow therapeutic windows are of particular concern.
These medications require precise delivery to maximize efficacy and minimize toxicity. Deviations from the recommended infusion rate can have significant consequences.
Where are Problems Occurring?
The issue is widespread across the United States, with reports emerging from hospitals of all sizes and types. Metropolitan areas and rural healthcare facilities alike are impacted.
No region is immune to the challenges posed by this medication delivery concern. The national scope of the problem demands a coordinated response.
When did This Issue Emerge?
While the practice of infusing 100 ml over 90 minutes has existed for some time, the increased attention and scrutiny are relatively recent. The rise of electronic health records (EHRs) and automated dispensing systems (ADS) played a role.
These technologies have made it easier to track medication administration and identify discrepancies. Increased reporting and analysis of adverse events have also contributed to the heightened awareness.
How is Medication Being Administered?
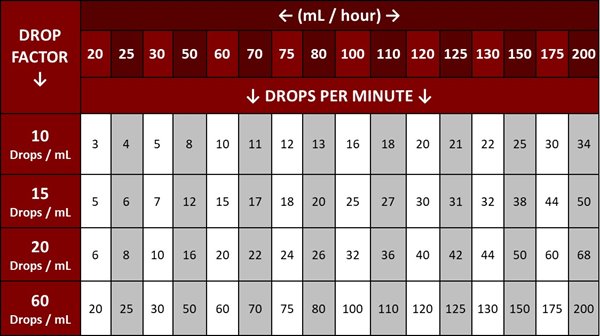
Medication is typically administered intravenously using infusion pumps or gravity-drip systems. The accuracy of these methods varies, with infusion pumps generally considered more precise.
Human error, such as incorrect programming of infusion pumps or miscalculation of drip rates, can also contribute to administration errors. This highlights the need for robust training and verification processes.
Immediate Actions and Future Directions
Hospitals are implementing several immediate actions to address the problem. These include reviewing and updating medication administration protocols to reflect best practices.
Retraining nursing staff on proper infusion techniques and emphasizing the importance of adherence to prescribed rates is also a priority. Implementing double-checks and independent verification procedures for high-risk medications is crucial.
Furthermore, healthcare organizations are enhancing their EHR systems to include alerts and reminders related to infusion rates. This ensures that clinicians are aware of the correct parameters at the point of care.
The FDA and other regulatory agencies are actively monitoring the situation and may issue further guidance. Pharmaceutical companies are also being encouraged to provide clearer and more concise instructions on their product labels.
Ongoing research and analysis are needed to better understand the factors contributing to medication administration errors. This includes examining the impact of different technologies and workflow processes.
The ultimate goal is to create a safer and more reliable medication delivery system that minimizes the risk of adverse events. This requires a collaborative effort involving hospitals, healthcare providers, regulatory agencies, and pharmaceutical companies.
The situation surrounding the 100 ml over 90 minutes infusion rate highlights the complexities of medication administration. Proactive measures are essential to protect patient safety and ensure optimal therapeutic outcomes. Further updates will be provided as the situation evolves.






.jpg)