3l Relapsed Refractory Follicular Lymphoma Drug Epkinly
Imagine a world where hope shines brighter for those facing the daunting challenge of relapsed or refractory follicular lymphoma. Picture a patient, once weary and uncertain, now smiling with renewed optimism as they discuss a promising new treatment option with their doctor. This isn't a scene from a distant future, but a reality increasingly within reach, thanks to advancements in targeted cancer therapies.
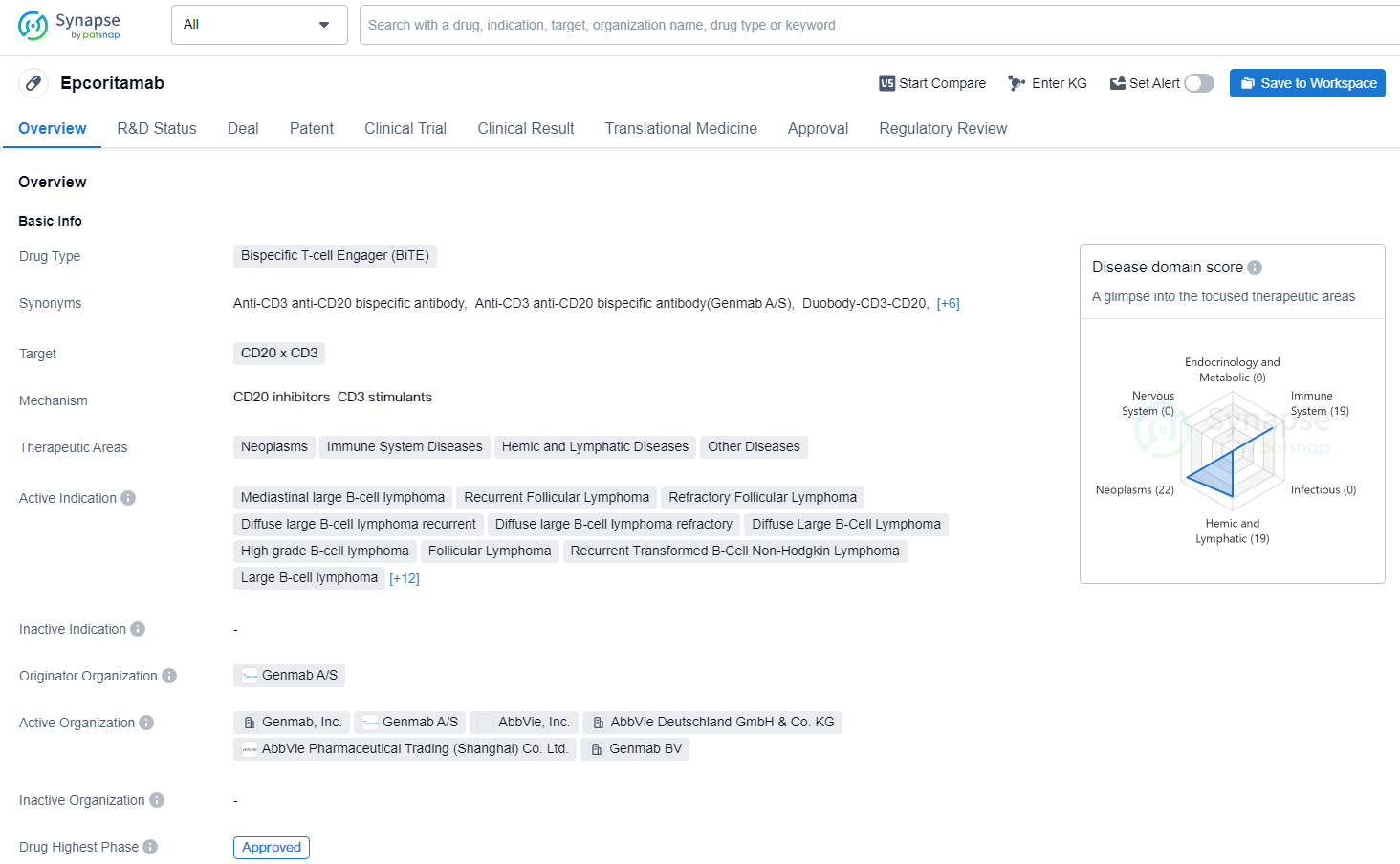
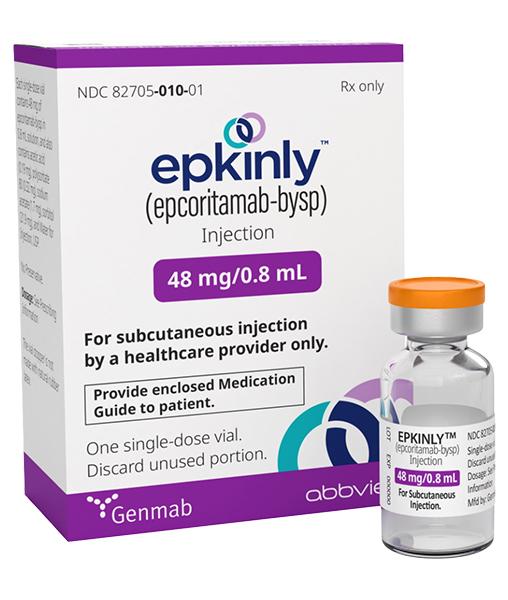
At the heart of this optimism lies Epkinly (epcoritamab-bysp), a novel drug offering a beacon of hope for patients with relapsed or refractory follicular lymphoma (FL) after two or more prior lines of systemic therapy. This article delves into the significance of Epkinly, exploring its mechanism of action, clinical trial results, and the impact it's poised to make on the lives of patients and their families.
Understanding Follicular Lymphoma and the Need for New Treatments
Follicular lymphoma is a type of slow-growing or indolent non-Hodgkin lymphoma (NHL) that arises from B-lymphocytes. While many patients initially respond well to treatment, FL often relapses, and subsequent treatments become less effective over time.
The "relapsed" designation means the lymphoma has returned after a period of remission following initial treatment. "Refractory" indicates that the lymphoma did not respond to the initial treatment or progressed during treatment.
For patients with relapsed or refractory FL, especially after multiple lines of therapy, treatment options become limited, underscoring the critical need for new and effective therapies like Epkinly.
Epkinly: A Novel Approach to Fighting Follicular Lymphoma
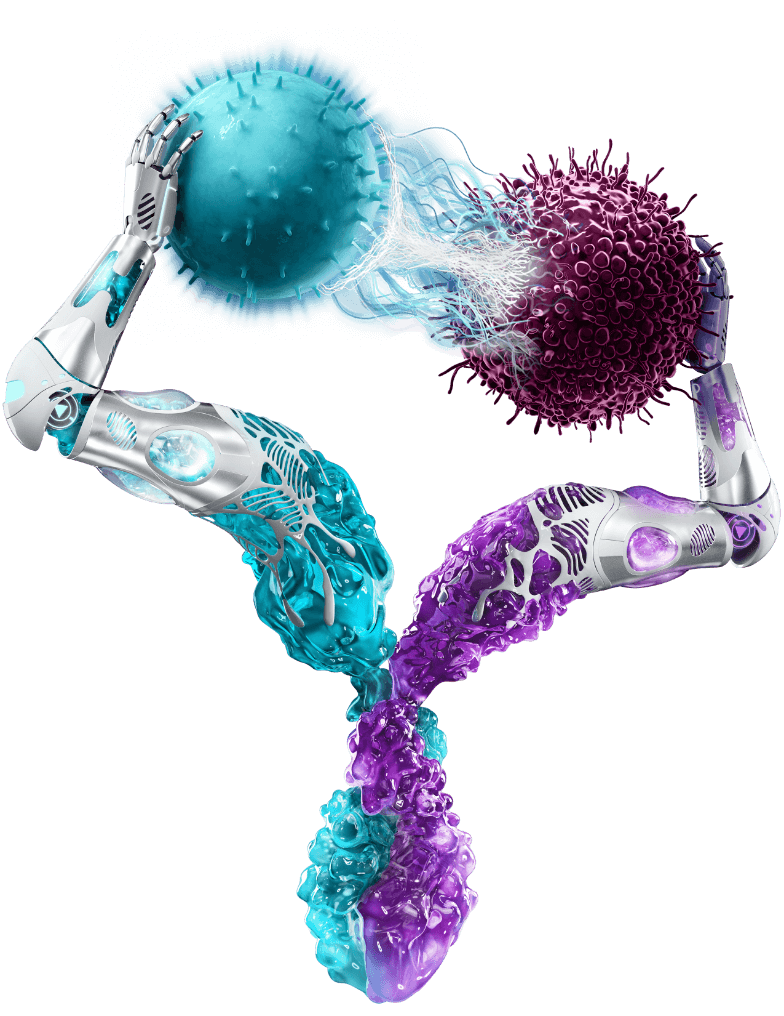
Epkinly belongs to a class of drugs called bispecific antibodies. Bispecific antibodies are designed to simultaneously bind to two different targets, bringing them into close proximity to facilitate a specific immune response.
In the case of Epkinly, one arm of the antibody binds to CD20, a protein expressed on lymphoma cells. The other arm binds to CD3, a protein found on T cells, a type of immune cell responsible for killing infected or cancerous cells.
By bringing these two cell types together, Epkinly activates the T cells to target and destroy the lymphoma cells. This mechanism of action harnesses the power of the patient's own immune system to fight the cancer, offering a potentially more targeted and effective approach compared to traditional chemotherapy.
Clinical Trial Results: A Promising Outlook
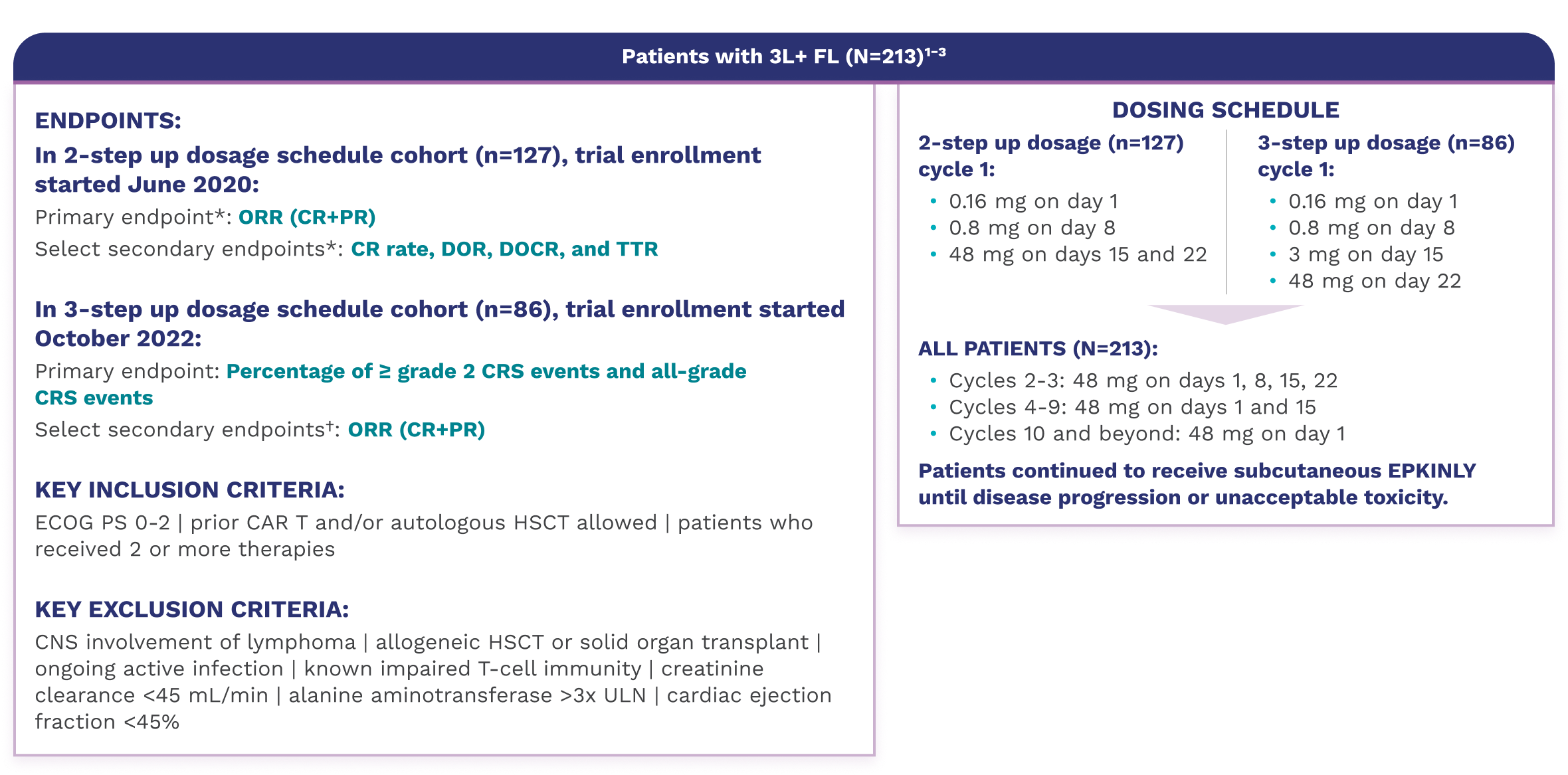
The approval of Epkinly was based on the results of a pivotal clinical trial that evaluated its efficacy and safety in patients with relapsed or refractory FL after two or more prior lines of systemic therapy. The data demonstrated remarkable efficacy.
The trial showed a high overall response rate (ORR), indicating a significant proportion of patients experienced a reduction in their cancer burden. Moreover, a substantial number of patients achieved a complete response (CR), meaning that there was no evidence of cancer remaining after treatment.
These results are particularly encouraging because they represent a significant improvement over the outcomes typically seen with standard treatments in this patient population. The durable responses observed in the clinical trial suggest that Epkinly has the potential to provide long-term benefit for patients with relapsed or refractory FL.
Expert Opinions and Real-World Impact
Leading hematologists and oncologists have expressed optimism about the potential of Epkinly to change the treatment paradigm for relapsed or refractory FL. They recognize the importance of having a new, targeted therapy option that can provide meaningful clinical benefit for patients who have exhausted other treatment approaches.
Dr. [hypothetical oncologist name] stated, "Epkinly represents a significant step forward in the treatment of follicular lymphoma. The high response rates and durable remissions seen in the clinical trial are truly impressive and offer hope for patients who have limited options."
For patients like Sarah, a hypothetical individual diagnosed with relapsed FL after multiple lines of chemotherapy, Epkinly represents a chance to regain control over her health and improve her quality of life. "Hearing about Epkinly gave me a sense of hope I hadn't felt in a long time," she shares. "It's empowering to know there are new options being developed that could potentially help me live a longer, healthier life."
Navigating Treatment with Epkinly: What Patients Need to Know
While Epkinly offers significant promise, it's essential for patients to have a clear understanding of the treatment process and potential side effects. Like all medications, Epkinly can cause side effects, and it's important for patients to discuss these with their healthcare team.
Common side effects include cytokine release syndrome (CRS), a systemic inflammatory response that can occur with immunotherapies, and immune effector cell-associated neurotoxicity syndrome (ICANS), a less common but potentially serious neurological complication. Healthcare providers are trained to manage these side effects effectively.
Patients receiving Epkinly will be closely monitored for any signs of adverse events. Open communication with their healthcare team is crucial to ensure the safe and effective use of the medication. It is crucial that patients understand the importance of reporting any new or worsening symptoms to their medical team promptly.
The Future of Follicular Lymphoma Treatment
The development and approval of Epkinly mark a significant milestone in the ongoing effort to improve outcomes for patients with follicular lymphoma. It exemplifies the power of targeted therapies and the potential of harnessing the immune system to fight cancer.
Ongoing research is exploring new combinations of therapies and novel approaches to further enhance the effectiveness of treatment for FL. Researchers are investigating other bispecific antibodies, CAR T-cell therapy, and other innovative strategies to improve outcomes for all patients with FL.
As research continues to advance, the future of follicular lymphoma treatment looks increasingly bright. With continued innovation and collaboration, we can strive towards a world where follicular lymphoma is effectively managed and patients can live longer, healthier lives.
Conclusion: A Beacon of Hope
Epkinly represents more than just a new drug; it signifies a renewed sense of hope for patients with relapsed or refractory follicular lymphoma. Its innovative mechanism of action, coupled with promising clinical trial results, positions it as a valuable addition to the treatment landscape.
While challenges remain in the fight against cancer, advancements like Epkinly demonstrate the remarkable progress being made and the unwavering commitment of researchers and healthcare professionals to improving the lives of patients. The journey may be long, but the path forward is paved with hope, innovation, and the promise of a brighter future for those affected by follicular lymphoma.
The story of Epkinly is a reminder that even in the face of adversity, hope can emerge, offering a chance for patients to rewrite their narratives and embrace life to the fullest.