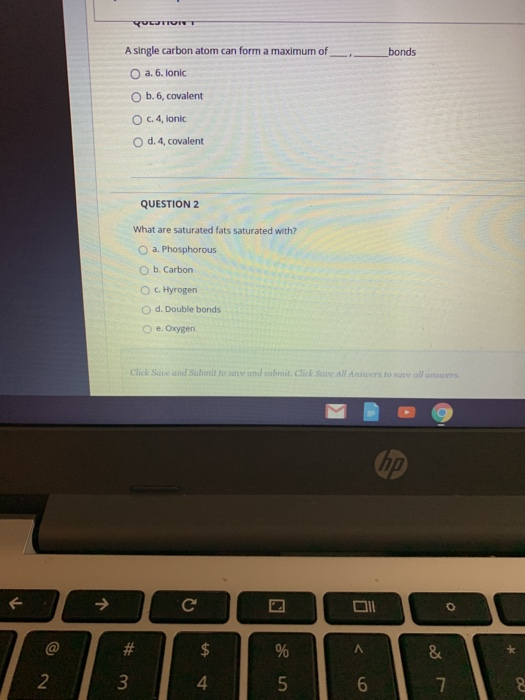
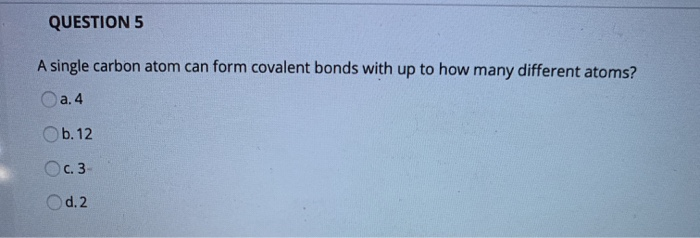
A Single Carbon Atom Can Form A Maximum Of

The seemingly simple assertion that a single carbon atom can form a maximum of four covalent bonds is a cornerstone of organic chemistry, underpinning our understanding of everything from the structure of DNA to the creation of advanced materials. While seemingly elementary, the implications of this tetravalency are profound, shaping the very nature of life and driving innovation across diverse scientific fields.
At its core, this concept, often taken for granted, dictates the architecture of the molecular world. Understanding the limitations and possibilities inherent in carbon's bonding capabilities is crucial for chemists, materials scientists, and biologists alike.
The Foundation of Organic Chemistry
The concept of carbon's tetravalency was first proposed by scientists like Friedrich August Kekulé in the mid-19th century. His insights revolutionized the understanding of organic compounds and laid the groundwork for modern organic chemistry.
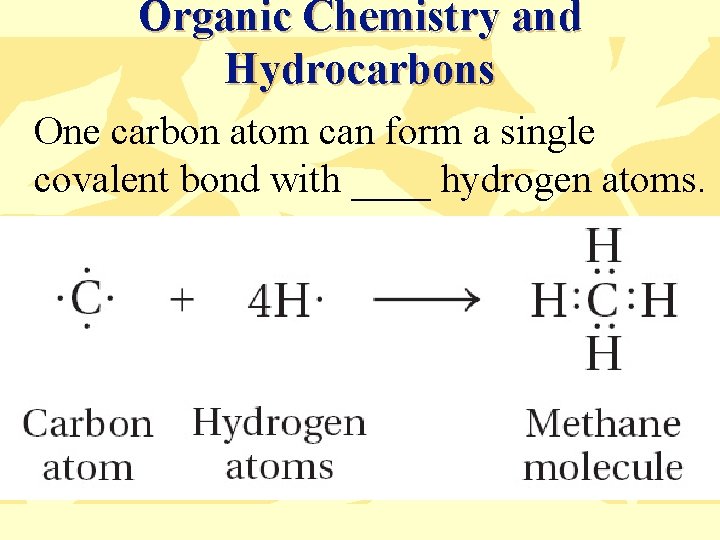
At the time, it was revolutionary to propose that a single carbon atom could form bonds with four other atoms. This principle allows for the creation of complex and diverse molecular structures, a defining characteristic of organic molecules.
The tetrahedral geometry around a carbon atom, dictated by these four bonds, is essential for understanding the three-dimensional structure of molecules. This spatial arrangement impacts a molecule's reactivity, physical properties, and biological function.
Why Four Bonds?
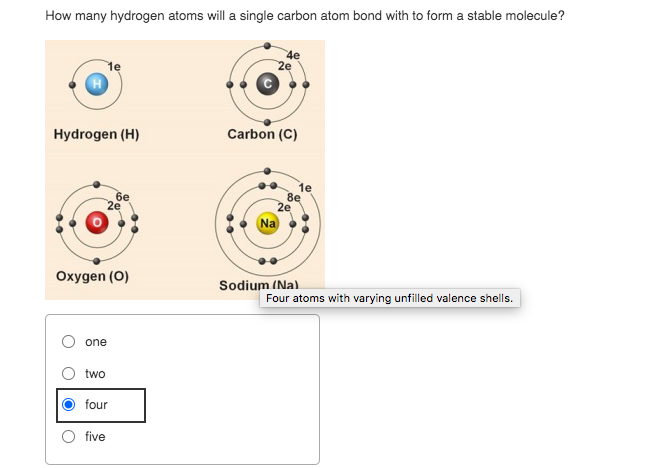
Carbon's electronic configuration is the key to its bonding behavior. With four valence electrons, it requires four more to achieve a stable octet, the configuration of a noble gas.
This "need" to complete its outer electron shell drives carbon to form four covalent bonds. These bonds can be single, double, or triple, allowing for even greater structural variety.
Other elements, like silicon, also possess four valence electrons. However, silicon's larger atomic size and weaker bonds make it less suitable as the backbone for complex molecular architectures, highlighting the unique suitability of carbon.
Implications Across Disciplines
In biology, carbon's tetravalency is fundamental to the structure of biomolecules like carbohydrates, lipids, proteins, and nucleic acids. The carbon-based backbone of these molecules provides the framework for life's essential processes.
The specific arrangement of carbon atoms in these molecules determines their unique functions. From the enzymatic activity of proteins to the storage of genetic information in DNA, carbon's bonding capabilities are crucial.
In materials science, the ability of carbon to form strong, stable bonds is exploited to create a wide range of materials. From polymers like polyethylene to advanced materials like carbon nanotubes and graphene, carbon's versatility is unparalleled.
Carbon Nanotubes and Graphene
Carbon nanotubes, cylindrical structures made of rolled-up graphene sheets, possess exceptional strength and electrical conductivity. These properties make them ideal for use in electronics, composites, and energy storage.
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, is another wonder material. Its incredible strength, flexibility, and conductivity have opened up possibilities for new technologies in electronics, sensors, and energy storage.
These materials owe their unique properties directly to the strong, stable bonds formed between carbon atoms. The arrangement of these bonds determines the overall structure and functionality of the material.
Beyond Traditional Bonding
While carbon typically forms four covalent bonds, there are rare exceptions. Some specialized carbon compounds can exhibit hypervalency, where a carbon atom appears to form more than four bonds.
However, these cases are typically stabilized by specific electronic or steric effects. They do not violate the fundamental principle that carbon's valence shell can only accommodate a certain number of electrons.
These exceptions are actively researched as they can potentially lead to the creation of novel materials with unusual properties. The field of research focuses on developing molecules which appear to violate the octet rule using elements that typically follow it.
A Constant in a Changing World
Despite advancements in chemistry and materials science, the principle that a single carbon atom can form a maximum of four covalent bonds remains a constant. It is a fundamental principle that underpins a vast array of scientific disciplines.
Understanding this basic concept allows researchers to design new molecules, develop innovative materials, and unravel the complexities of life itself.
As our understanding of chemistry deepens, this principle will continue to serve as a guiding light, shaping the future of science and technology. The simplicity of this tenet will continue to drive innovation in the realms of chemistry, material science and biology.