Anpd001 Aspen Neuroscience A Phase 1/2a Clinical Trial Update 2024

The relentless pursuit of effective treatments for Parkinson's disease, a debilitating neurodegenerative disorder affecting millions worldwide, has taken a significant turn. A recent update on a Phase 1/2a clinical trial involving ANPD001, a cell therapy developed by Aspen Neuroscience, is generating considerable buzz within the medical community and offering renewed hope to patients and their families. The data, while preliminary, suggest a potential path forward in addressing the underlying causes of this challenging condition.
This article delves into the details of the Aspen Neuroscience ANPD001 Phase 1/2a clinical trial update presented in 2024. It examines the therapy's mechanism of action, the trial design, key findings, potential implications, and the challenges that lie ahead. The aim is to provide a comprehensive overview of this promising development in Parkinson's disease research, placing it within the broader context of ongoing efforts to combat this devastating illness.
Understanding ANPD001 and its Mechanism
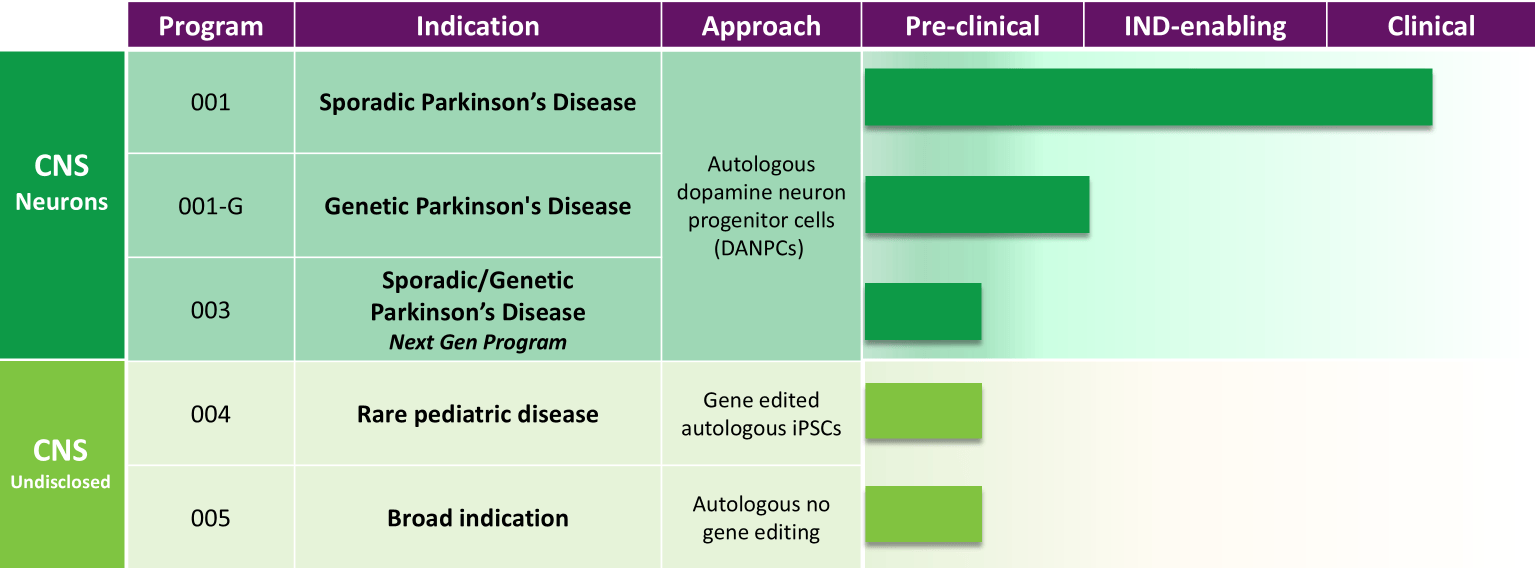
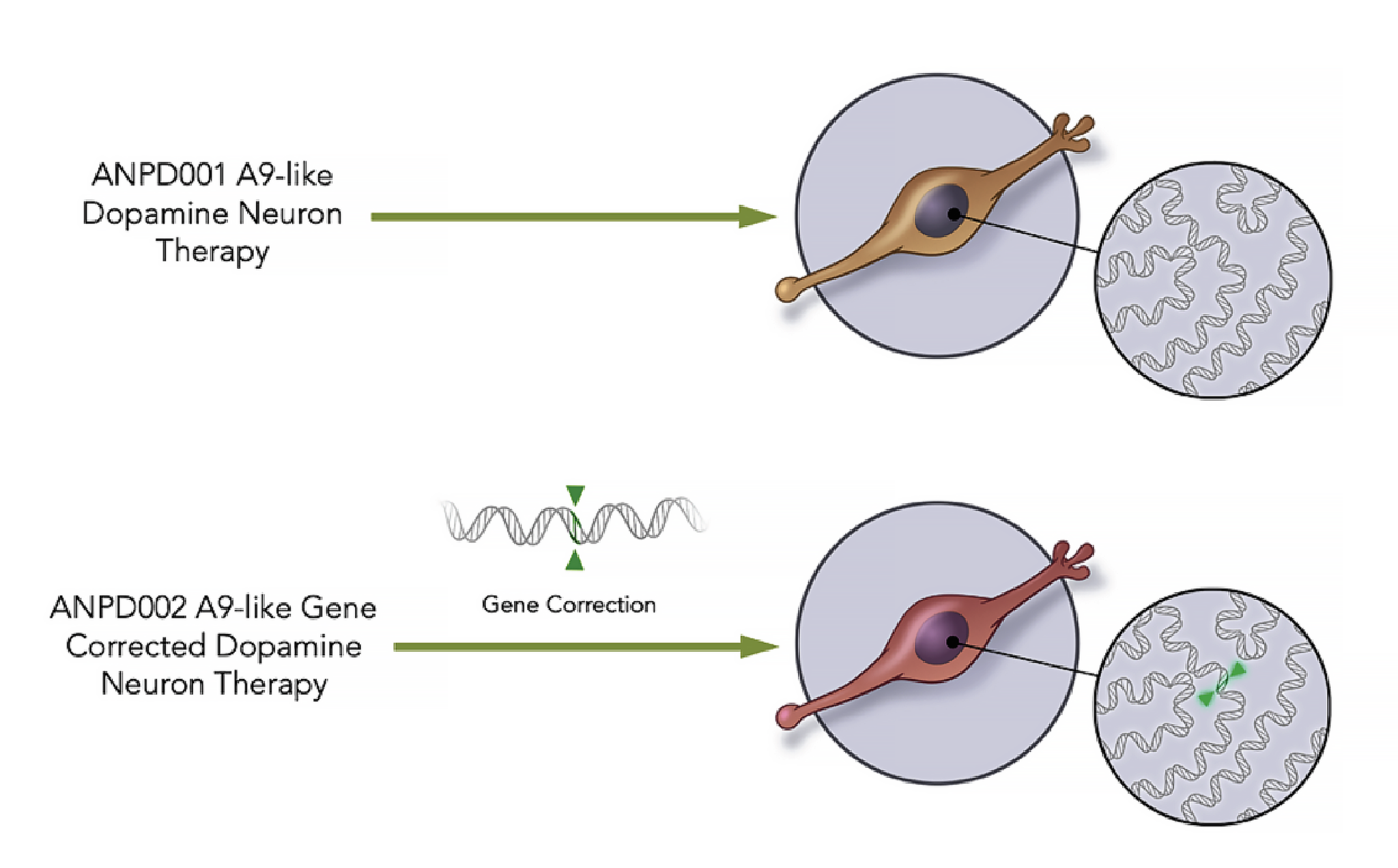
ANPD001 is an autologous cell therapy derived from a patient's own skin cells. These cells are reprogrammed into induced pluripotent stem cells (iPSCs) and then differentiated into dopamine-producing neurons, the very cells that are lost in Parkinson's disease.
The therapy aims to replace these lost neurons, thereby restoring dopamine levels in the brain and alleviating motor symptoms associated with the disease. This approach represents a significant departure from symptomatic treatments, which only mask the symptoms without addressing the underlying neurodegeneration.
Aspen Neuroscience emphasizes the importance of using autologous cells to minimize the risk of immune rejection. This personalization is a key aspect of the ANPD001 strategy.
Phase 1/2a Clinical Trial Design
The Phase 1/2a clinical trial is designed to assess the safety, tolerability, and preliminary efficacy of ANPD001 in patients with moderate to severe Parkinson's disease. The trial is an open-label study, meaning that both the researchers and the participants know who is receiving the treatment.
Patients enrolled in the trial underwent a thorough screening process to ensure they met specific eligibility criteria. The therapy is administered via stereotactic neurosurgery, a precise technique that allows for the accurate placement of cells into the target region of the brain, the putamen.
Participants are closely monitored for adverse events and undergo regular neurological assessments to evaluate changes in motor function, cognitive abilities, and overall quality of life.
Key Findings from the 2024 Update
The 2024 update on the Phase 1/2a trial presented encouraging, though preliminary, data regarding the safety and tolerability of ANPD001. No serious adverse events related to the cell therapy have been reported thus far.
The initial data suggest that the transplanted cells are surviving and integrating into the brain tissue. Evidence of dopamine production from the transplanted neurons has also been observed through imaging techniques.
While the primary endpoint of the Phase 1/2a trial is safety, early indications of clinical improvement have been noted in some patients. These improvements include slight enhancements in motor function scores and a reduction in the need for dopaminergic medications.
Potential Implications and Future Directions
The positive preliminary findings from the Aspen Neuroscience ANPD001 trial have significant implications for the future of Parkinson's disease treatment. If these results are confirmed in larger, controlled studies, cell therapy could become a viable option for slowing down or even reversing the progression of the disease.
The success of this trial could also pave the way for the development of similar cell-based therapies for other neurodegenerative disorders, such as Alzheimer's disease and Huntington's disease. This could revolutionize the treatment landscape for these devastating conditions.
Aspen Neuroscience plans to continue to follow the patients in the Phase 1/2a trial for a longer period to assess the long-term safety and efficacy of ANPD001. They are also planning to initiate a Phase 2b randomized, controlled trial to further evaluate the therapy's effectiveness.
Challenges and Considerations
Despite the promising results, significant challenges remain in the development of cell therapies for Parkinson's disease. One of the major challenges is ensuring the long-term survival and functionality of the transplanted cells.
Another challenge is optimizing the delivery method to ensure that the cells are accurately placed and effectively integrated into the brain tissue. Furthermore, the high cost of cell therapies could limit their accessibility to patients.
Ethical considerations surrounding the use of stem cells and gene editing also need to be carefully addressed. These must be addressed through ongoing dialogue and rigorous scientific oversight.
Expert Perspectives
According to Dr. Maria Garcia, a leading neurologist specializing in Parkinson's disease, "The Aspen Neuroscience ANPD001 trial represents a significant step forward in our quest for disease-modifying therapies." She cautioned that the data are still preliminary, but emphasized the importance of continued research in this area.
Dr. David Lee, a neurosurgeon with extensive experience in cell transplantation, added, "The precise surgical delivery of cells is crucial for the success of these therapies." He highlighted the need for advanced imaging techniques and refined surgical procedures to optimize cell placement.
Patient advocacy groups have also expressed cautious optimism about the ANPD001 trial. They emphasize the importance of including patients in the research process and ensuring that new therapies are accessible to all who need them.
Conclusion
The Aspen Neuroscience ANPD001 Phase 1/2a clinical trial update provides a glimpse of hope in the ongoing battle against Parkinson's disease. While much work remains to be done, the preliminary data suggest that cell therapy has the potential to transform the treatment of this debilitating condition.
The future of Parkinson's disease research hinges on continued innovation, rigorous clinical trials, and a collaborative approach involving scientists, clinicians, patients, and advocacy groups. The development of disease-modifying therapies like ANPD001 represents a critical step towards improving the lives of millions affected by this devastating illness.
Further investigation and long-term follow-up are essential to fully assess the potential of ANPD001. Continued funding and unwavering dedication are vital to moving forward in the quest for a cure.