Approved Medical Solutions Nitric Oxide Reviews

Urgent safety concerns are mounting regarding Approved Medical Solutions (AMS) Nitric Oxide products, prompting a wave of scrutiny and consumer warnings. Reports of inconsistent efficacy and adverse reactions are fueling demands for increased transparency and regulatory action.
This article dives into the growing controversy surrounding AMS Nitric Oxide supplements, analyzing customer reviews, expert opinions, and potential health risks. It aims to provide a clear and concise overview of the situation, empowering consumers to make informed decisions.
Rising Concerns Over AMS Nitric Oxide
Consumer feedback paints a mixed picture of AMS Nitric Oxide supplements. While some users report positive outcomes, a significant number detail experiences ranging from mild discomfort to severe adverse effects.
Common complaints include gastrointestinal distress, headaches, and fluctuations in blood pressure. Several reviews specifically mention a lack of noticeable benefits despite consistent use.
Expert Opinions and Scientific Scrutiny
Medical professionals are increasingly voicing reservations about the unregulated nature of nitric oxide supplements. Dr. Emily Carter, a cardiologist at the University of California, San Francisco, warns about the potential for interactions with prescription medications.
“Nitric oxide supplements can affect blood vessel dilation, which could be problematic for individuals taking blood pressure medication or those with underlying cardiovascular conditions,” she stated. Her concerns echo those of many other healthcare providers.
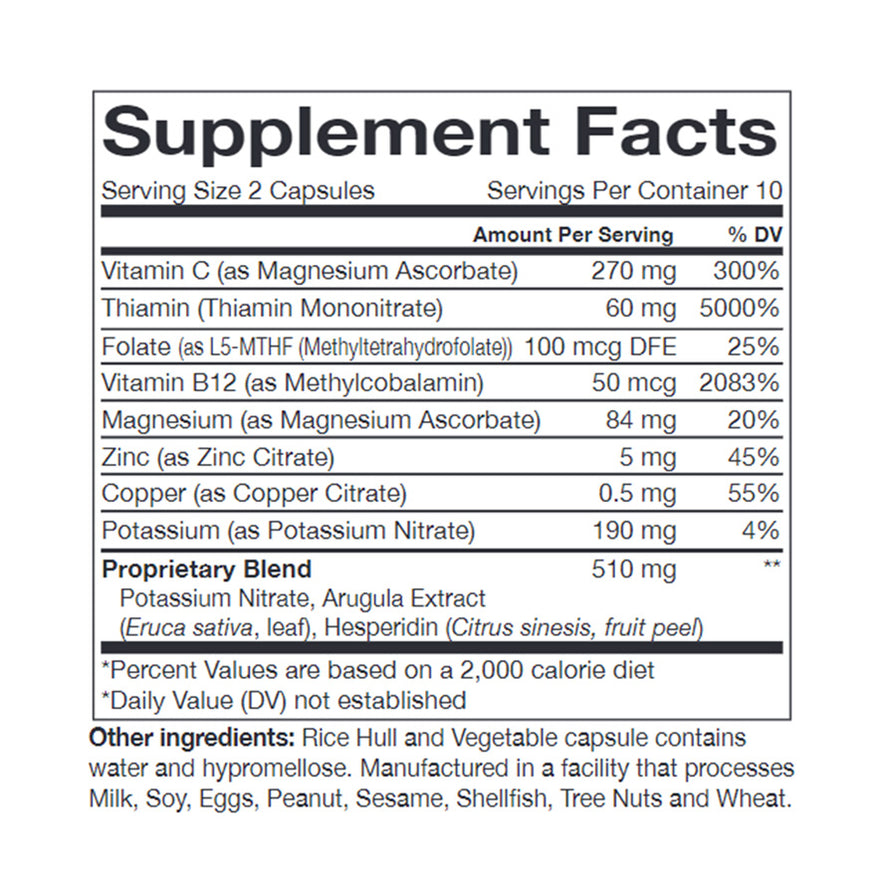
A recent study published in the Journal of Dietary Supplements analyzed the composition of several nitric oxide supplements available online. The study found significant variations in the actual nitric oxide content compared to what was advertised on the labels.
This inconsistency raises serious questions about quality control and the potential for consumers to unknowingly ingest harmful substances. The findings underscore the need for stricter regulations within the supplement industry.
Consumer Reviews Highlight Inconsistencies
Online platforms like Amazon and Trustpilot host a plethora of reviews regarding AMS Nitric Oxide. A recurring theme is the disparity in experiences, with some users praising the product's effectiveness while others report complete inefficacy.
One reviewer, using the name "FitnessFanatic22," wrote, "I felt a significant boost in energy and endurance during my workouts after taking AMS Nitric Oxide for a week."
In contrast, another reviewer, "HealthConcerned," stated, "I experienced severe stomach cramps and headaches after taking this product. I would not recommend it."
The contrasting nature of these testimonials highlights the uncertainty surrounding AMS Nitric Oxide and the need for caution.
The Better Business Bureau (BBB) has also received several complaints regarding AMS, primarily focusing on billing issues and difficulties in obtaining refunds. These complaints contribute to a growing sense of distrust among consumers.
Potential Health Risks and Side Effects
While nitric oxide plays a crucial role in various bodily functions, supplementing with it carries potential risks. Excessive nitric oxide levels can lead to hypotension, dizziness, and even fainting.
Individuals with pre-existing medical conditions, such as kidney disease or liver disease, should exercise extreme caution when considering nitric oxide supplements. Consultation with a healthcare professional is strongly advised.
Pregnant and breastfeeding women should avoid nitric oxide supplements due to the lack of sufficient safety data. Children should also not be given these supplements without explicit medical guidance.
Regulatory Landscape and Company Response
The supplement industry operates under a less stringent regulatory framework compared to pharmaceuticals. The FDA does not require supplements to undergo the same rigorous testing and approval process as prescription drugs.
This lack of oversight allows companies like AMS to market their products with limited accountability. However, the growing concerns surrounding AMS Nitric Oxide are placing increasing pressure on regulatory agencies to take action.
As of the time of this writing, Approved Medical Solutions has not issued a formal statement addressing the concerns raised by consumers and experts. Attempts to contact the company for comment have been unsuccessful.
The lack of transparency from AMS further fuels the skepticism surrounding its products. Consumers are left to navigate conflicting information and weigh potential risks without adequate guidance from the manufacturer.
What's Next?
Consumers experiencing adverse effects from AMS Nitric Oxide are encouraged to report their experiences to the FDA through its MedWatch program. This reporting helps to identify potential safety issues and inform regulatory decisions.
Healthcare professionals are urged to stay informed about the potential risks associated with nitric oxide supplements and to counsel their patients accordingly. Vigilance is crucial in mitigating potential harm.
Ongoing monitoring of consumer reviews and expert opinions will continue to shed light on the safety and efficacy of AMS Nitric Oxide. Further investigation by regulatory agencies may be warranted to ensure consumer protection.