Building Vocabulary Components Of An Atom

Imagine holding a single grain of sand. In that seemingly simple speck lies a universe of complexity, a hidden world of particles buzzing with energy and bound together by invisible forces. This is the essence of the atom, the fundamental building block of everything around us, and understanding its structure is key to unlocking the secrets of the universe.
Atoms, though once believed to be indivisible, are composed of even smaller particles. Understanding these particles, how they interact, and how we name and describe them, is crucial for grasping the fundamentals of chemistry and physics. This article will explore the components of an atom, while also introducing some helpful vocabulary to navigate the world of atomic structure.
The Historical Perspective
The concept of the atom has evolved over millennia. Ancient Greek philosophers, like Democritus, first proposed the idea of indivisible particles as the foundation of matter. However, this was purely philosophical, lacking any experimental evidence.
It wasn't until the early 19th century that John Dalton provided a scientific basis for the atomic theory. His experiments suggested that all matter is composed of atoms, which are indivisible and indestructible in chemical reactions. Dalton also posited that all atoms of a given element are identical.
While Dalton's model laid the groundwork, it was soon discovered that atoms *are* in fact divisible.
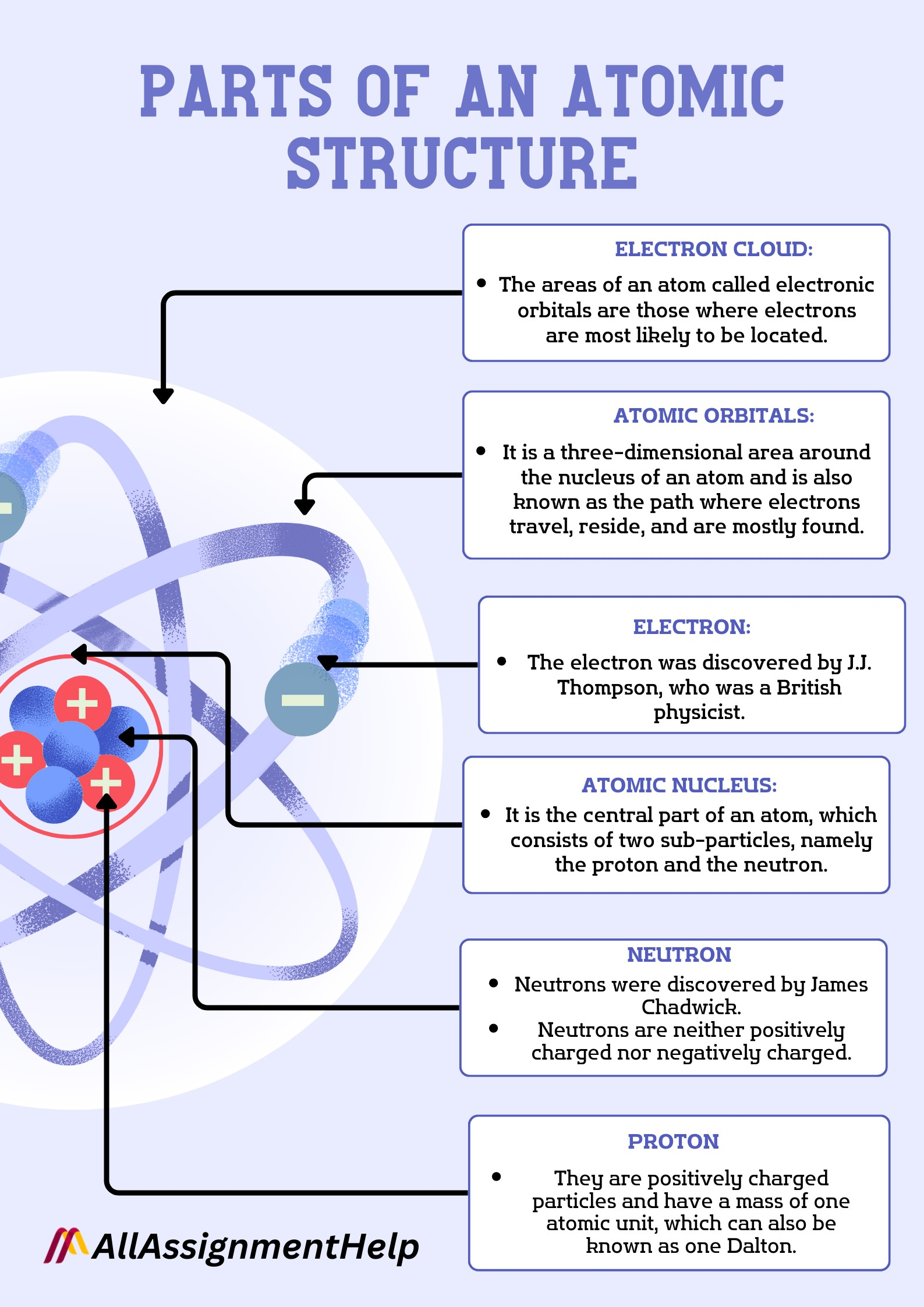
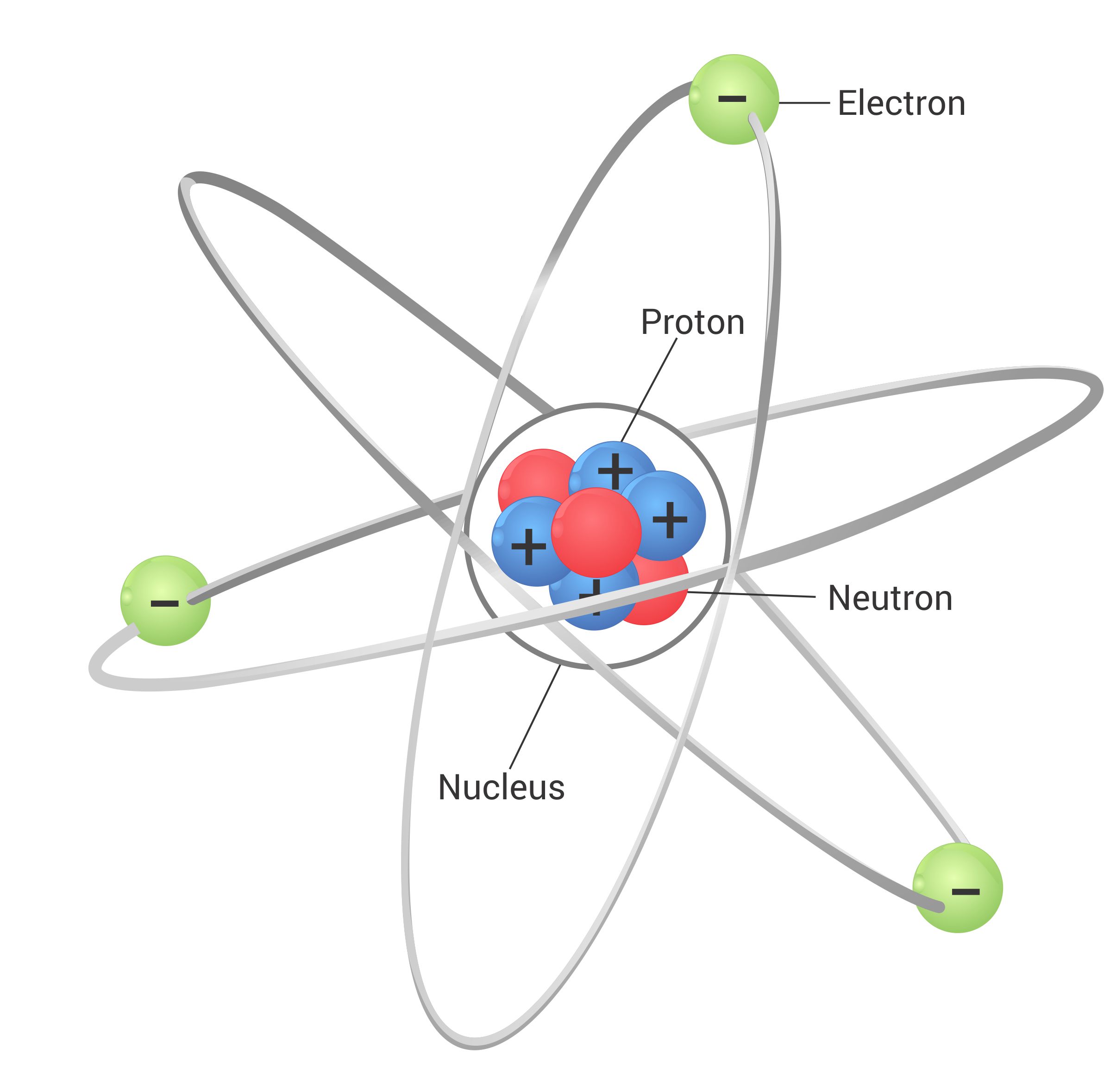
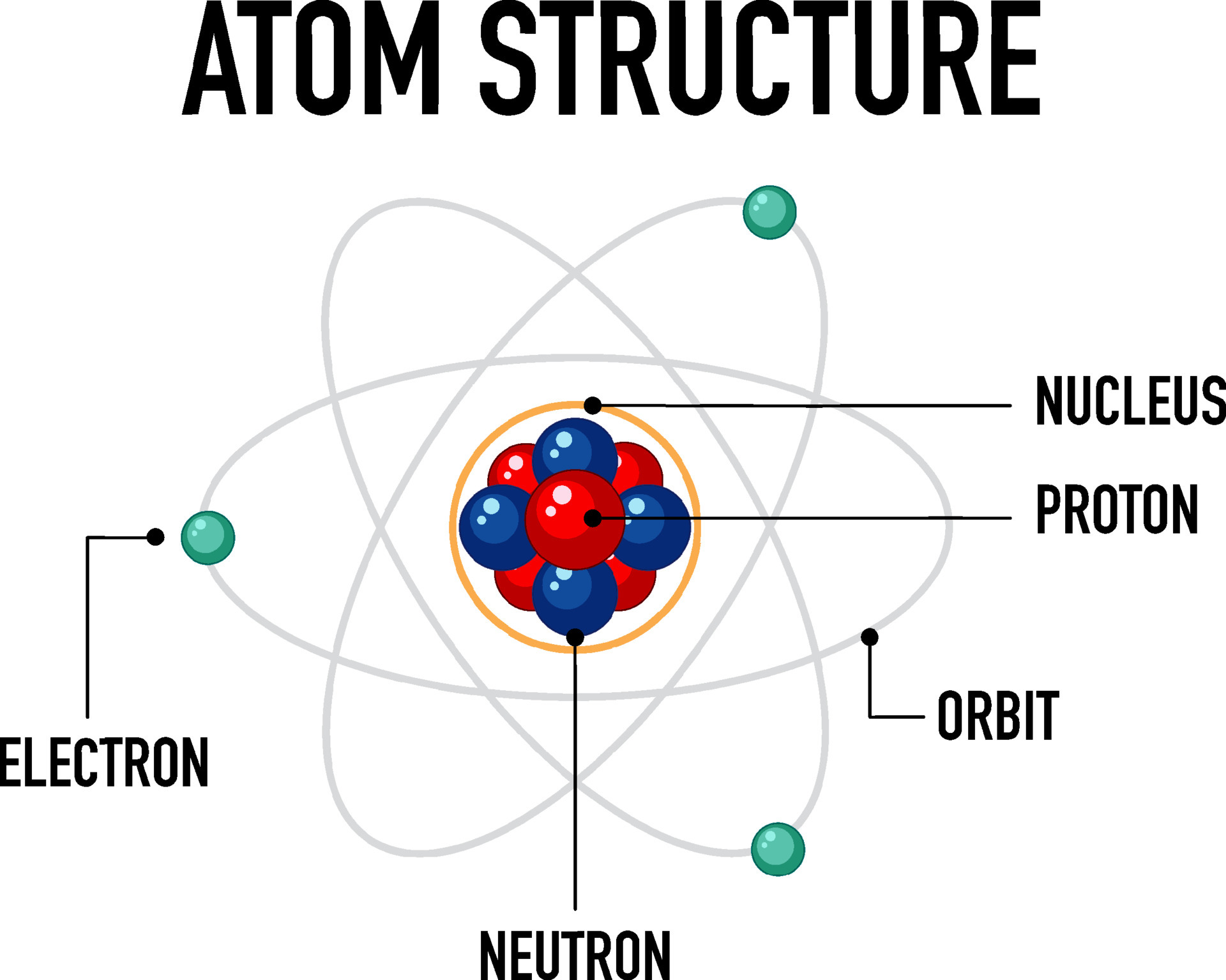
The Three Main Players: Protons, Neutrons, and Electrons
Protons: The Positively Charged Identity Markers
The discovery of the electron by J.J. Thomson in 1897 shook the foundation of Dalton's indivisible atom. Thomson's "plum pudding model" proposed that atoms were positively charged spheres with negatively charged electrons embedded within.
However, Ernest Rutherford's gold foil experiment in 1911 revolutionized our understanding. Rutherford discovered the nucleus, a tiny, dense, positively charged core at the center of the atom. He proposed that most of the atom's mass and all of its positive charge were concentrated in this central region.
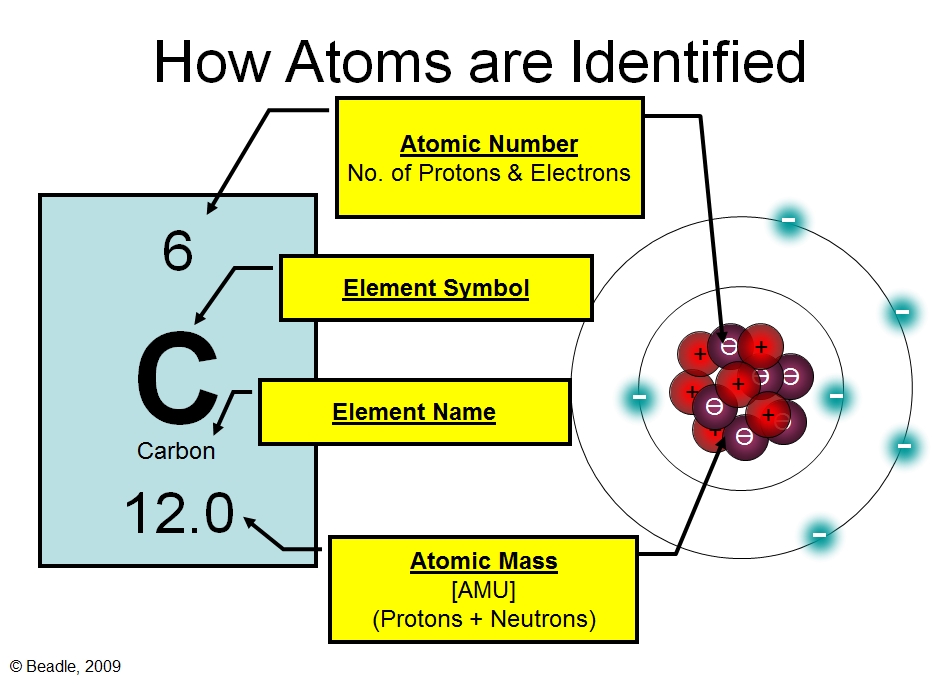
This positive charge is due to the presence of protons, subatomic particles with a positive charge. The number of protons in the nucleus defines the element; for example, all atoms with one proton are hydrogen, all with six are carbon, and so on. This number is known as the *atomic number*, a fundamental property of each element.
"Every science begins as philosophy and ends as art." - Will Durant
Neutrons: The Neutral Stabilizers
The mystery of nuclear stability led to the discovery of the neutron by James Chadwick in 1932. Neutrons are neutral particles found in the nucleus along with protons. They contribute to the atom's mass but do not affect its charge.
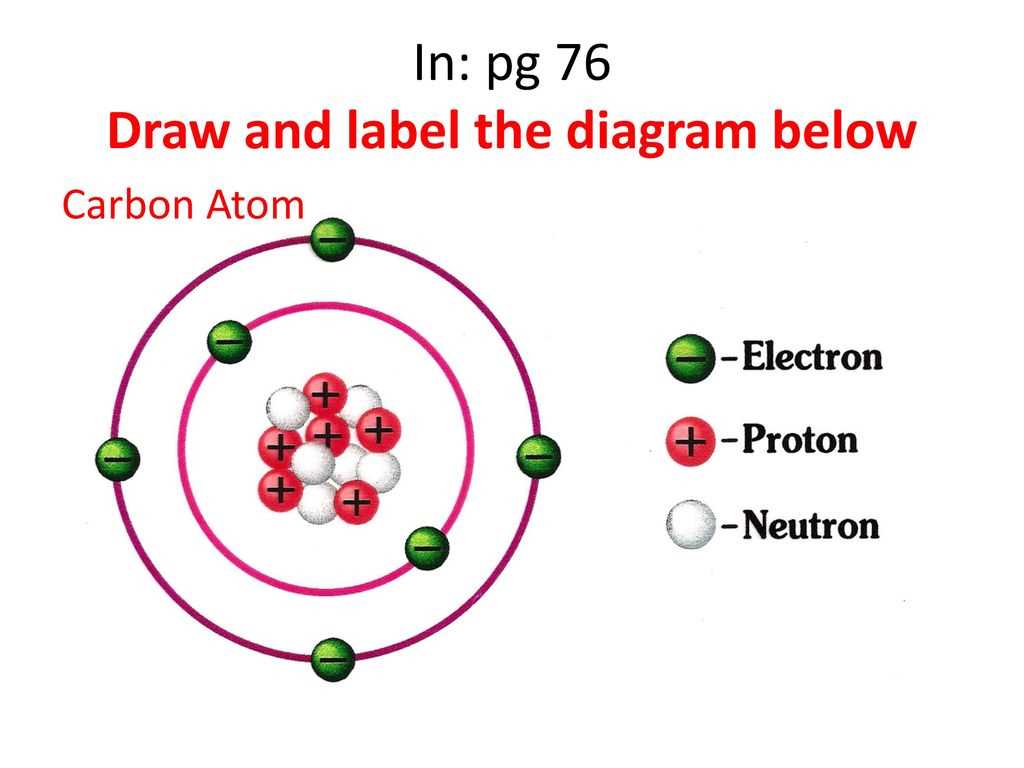
The number of neutrons can vary within a single element, leading to the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. For example, carbon-12 (12C) has 6 protons and 6 neutrons, while carbon-14 (14C) has 6 protons and 8 neutrons.
These variations in neutron number impact the atomic mass, leading to the concept of *atomic mass number* which is the sum of protons and neutrons in an atom's nucleus. Atomic mass is typically measured in atomic mass units (amu).
Electrons: The Negatively Charged Orbiters
Electrons, discovered by J.J. Thomson, are negatively charged particles that orbit the nucleus. They are much lighter than protons and neutrons, contributing very little to the atom's overall mass.
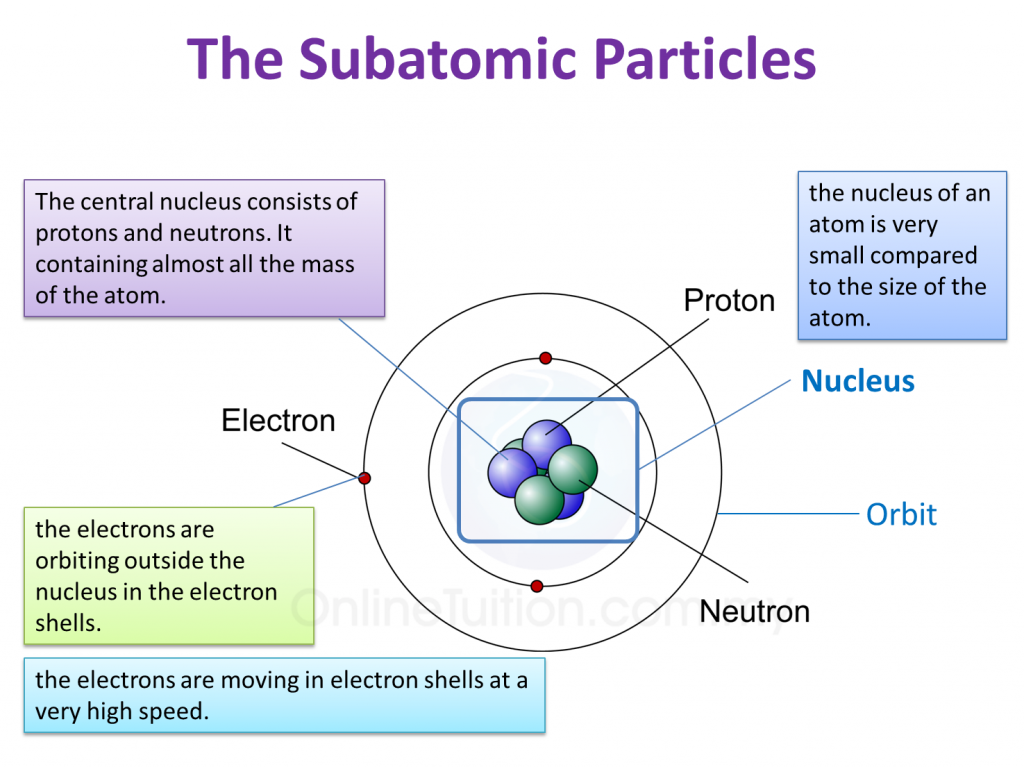
Electrons exist in specific energy levels or *shells* around the nucleus. The arrangement of electrons in these shells determines the chemical properties of the element. The outermost shell, known as the *valence shell*, is particularly important as it dictates how an atom interacts with other atoms to form chemical bonds.
The number of electrons in a neutral atom is equal to the number of protons in the nucleus. If an atom gains or loses electrons, it becomes an *ion*, carrying a net electrical charge. A positively charged ion is called a *cation*, while a negatively charged ion is called an *anion*.
Vocabulary Building Blocks
Understanding the structure of an atom comes with its own specialized vocabulary. Here are some key terms to help you navigate this field:
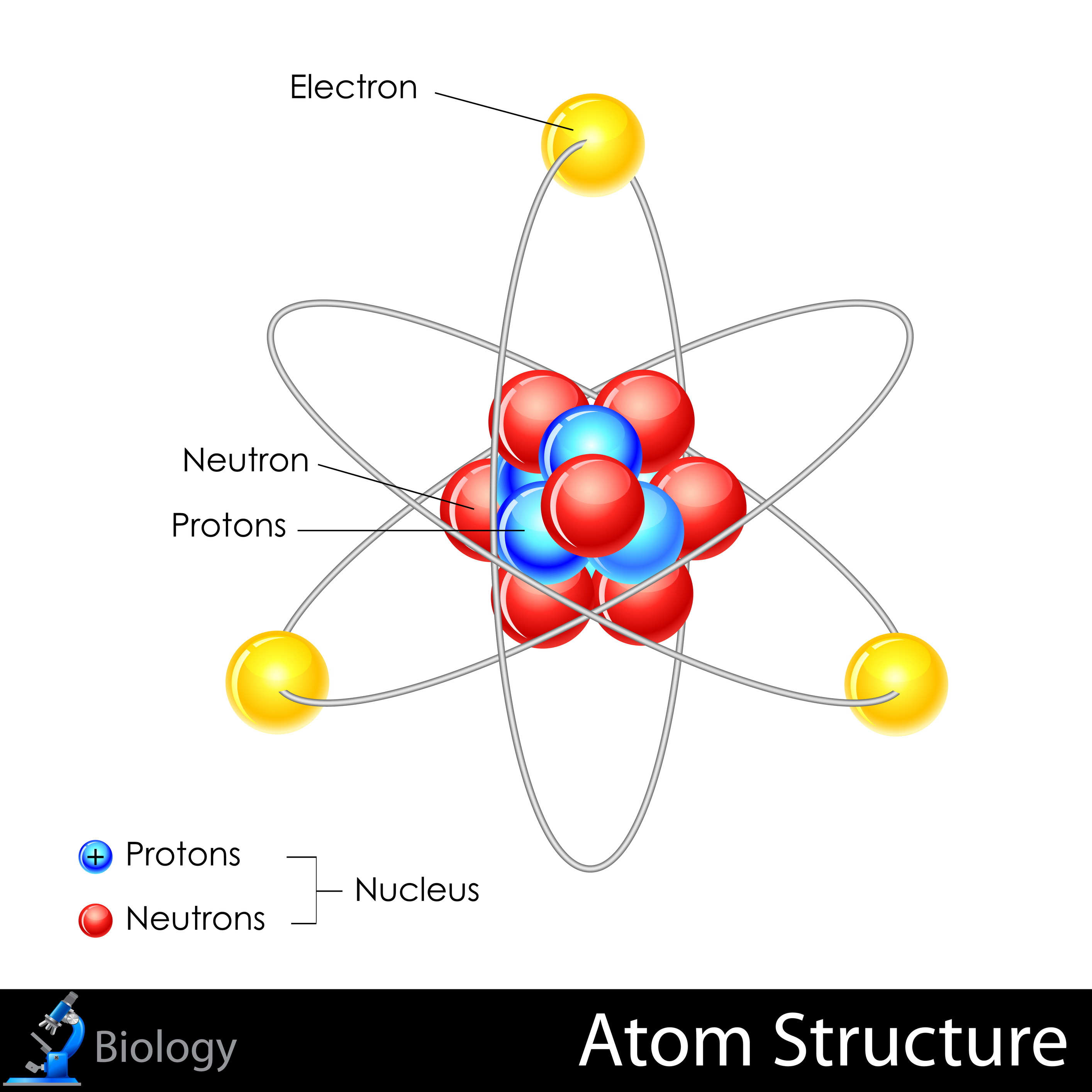
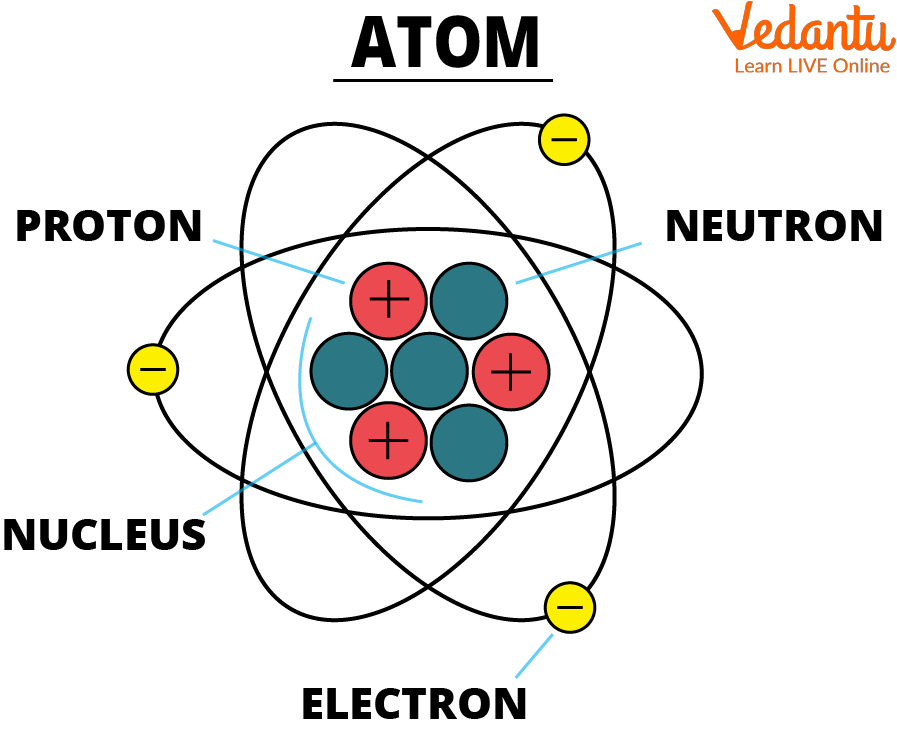
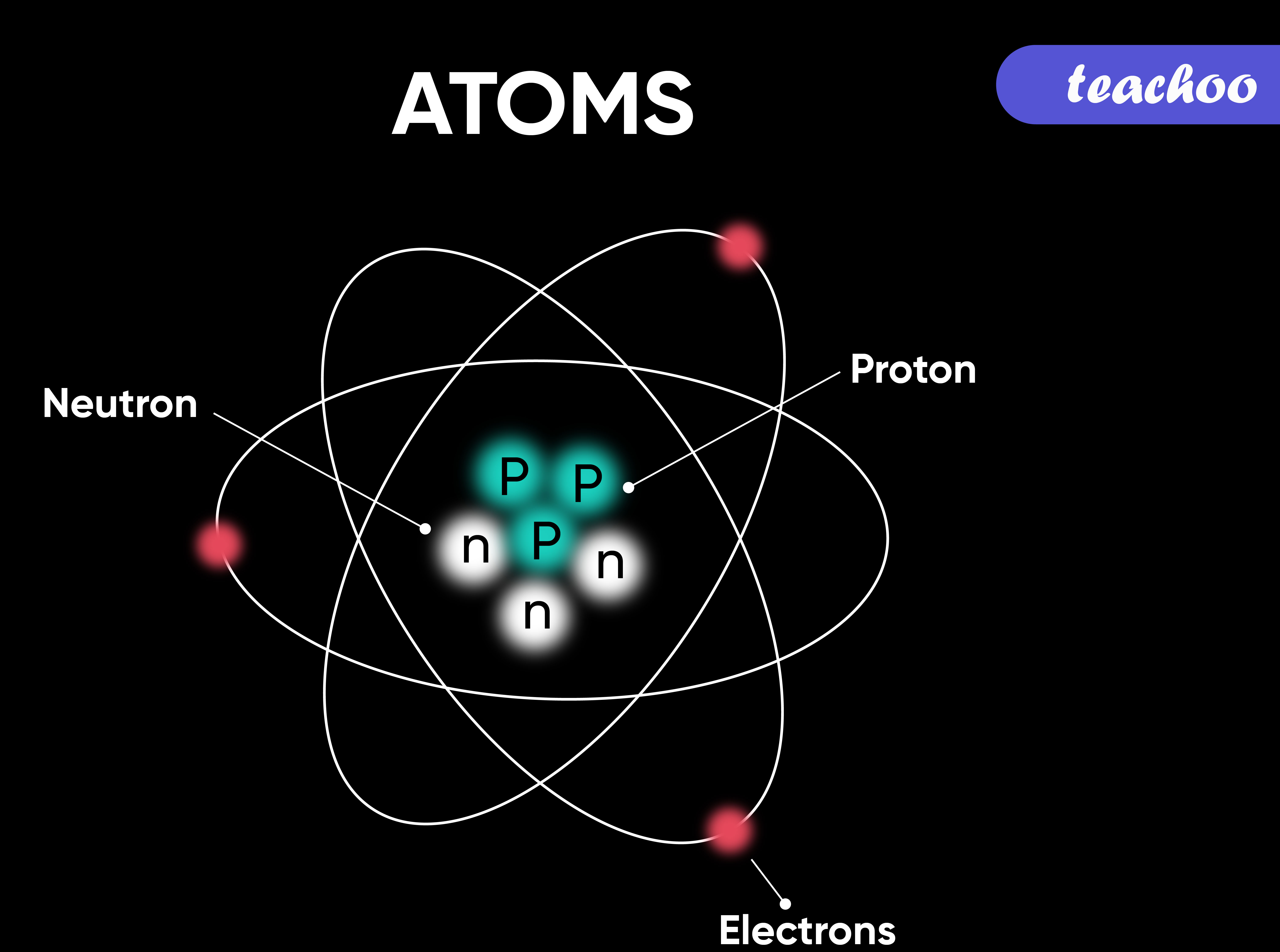
- Atom: The basic building block of matter, consisting of protons, neutrons, and electrons.
- Proton: A positively charged particle found in the nucleus of an atom.
- Neutron: A neutral particle found in the nucleus of an atom.
- Electron: A negatively charged particle that orbits the nucleus of an atom.
- Nucleus: The dense, positively charged core of an atom, containing protons and neutrons.
- Atomic Number: The number of protons in the nucleus of an atom, which defines the element.
- Mass Number: The total number of protons and neutrons in the nucleus of an atom.
- Isotope: Atoms of the same element with the same number of protons but different numbers of neutrons.
- Ion: An atom or molecule that has gained or lost electrons, giving it a net electrical charge.
- Cation: A positively charged ion.
- Anion: A negatively charged ion.
- Valence Shell: The outermost electron shell of an atom, which determines its chemical properties.
Beyond the Basics: Quarks and Leptons
The story doesn't end with protons, neutrons, and electrons. These particles are themselves composed of even smaller entities. For example, protons and neutrons are made up of *quarks*, fundamental particles that are held together by the strong nuclear force.
Electrons, on the other hand, are *leptons*, another type of fundamental particle. The exploration of these subatomic particles is the domain of particle physics, pushing the boundaries of our understanding of matter and energy.
Significance and Applications
Understanding the components of an atom is not just an academic exercise. It has profound implications for numerous fields, including medicine, materials science, and energy production. Radioactive isotopes, for example, are used in medical imaging and cancer therapy. The properties of materials are directly linked to their atomic structure and bonding.
The development of nuclear energy relies on our understanding of nuclear reactions, which involve changes in the nucleus of an atom. Moreover, advancements in nanotechnology are driven by our ability to manipulate matter at the atomic level.
The constant refinement of our understanding of atomic structure allows for continuous innovations that shape our modern world.
A Continuing Journey
The journey to understand the atom has been a long and winding one, filled with brilliant minds and groundbreaking experiments. It continues today with ongoing research into the fundamental constituents of matter and the forces that govern their interactions.
While we have come a long way since the ancient Greeks, there are still many mysteries to unravel. The quest to understand the atom, the universe's fundamental building block, is an ongoing adventure that promises to reveal even deeper insights into the nature of reality.
So, the next time you see a grain of sand, remember the complex and fascinating world hidden within. It's a reminder that even the smallest things can hold the greatest secrets, waiting to be discovered by curious minds.