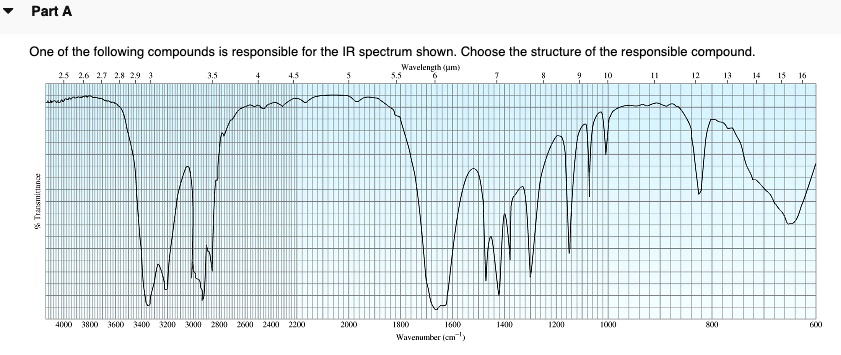
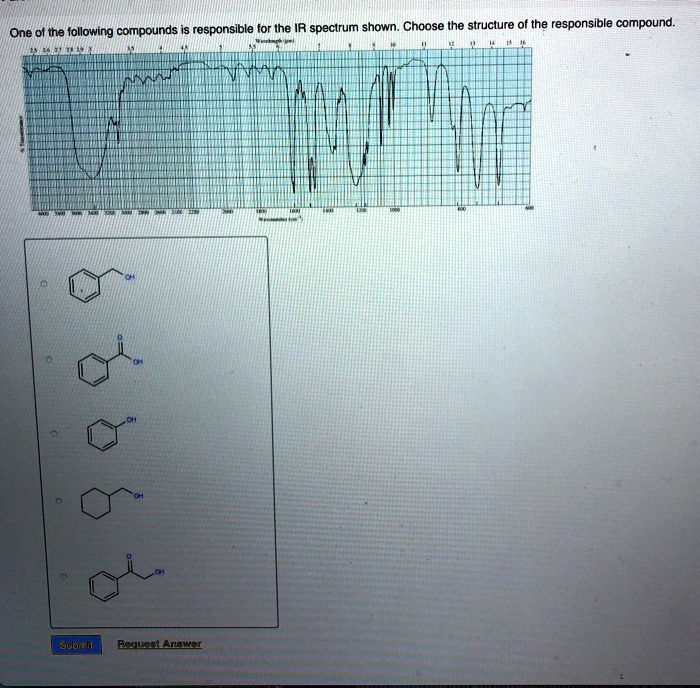
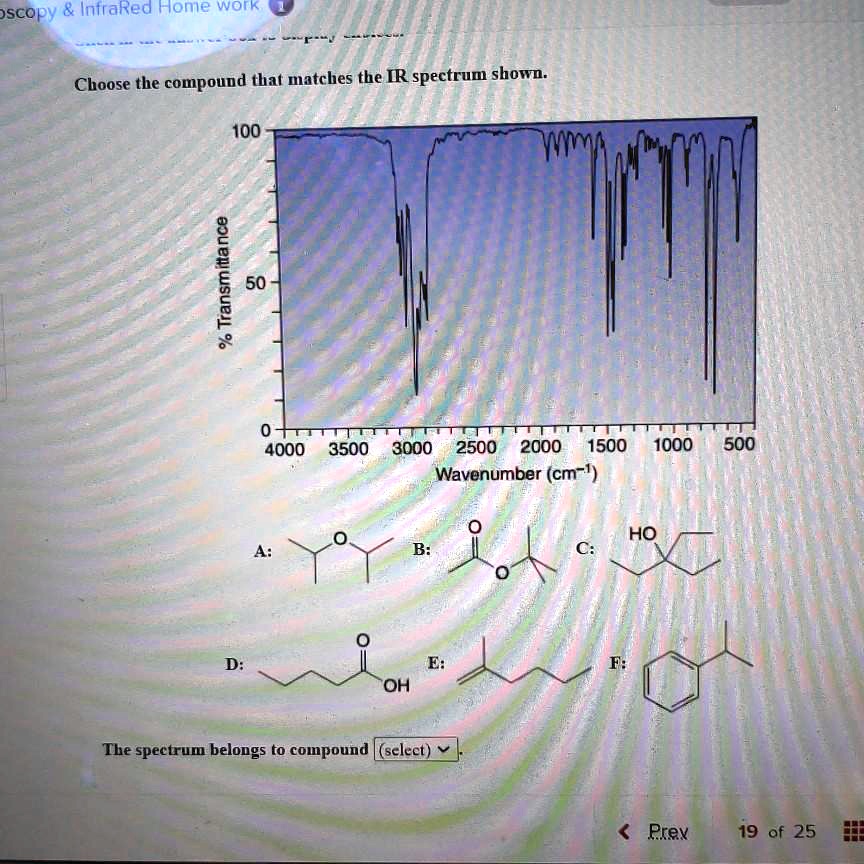
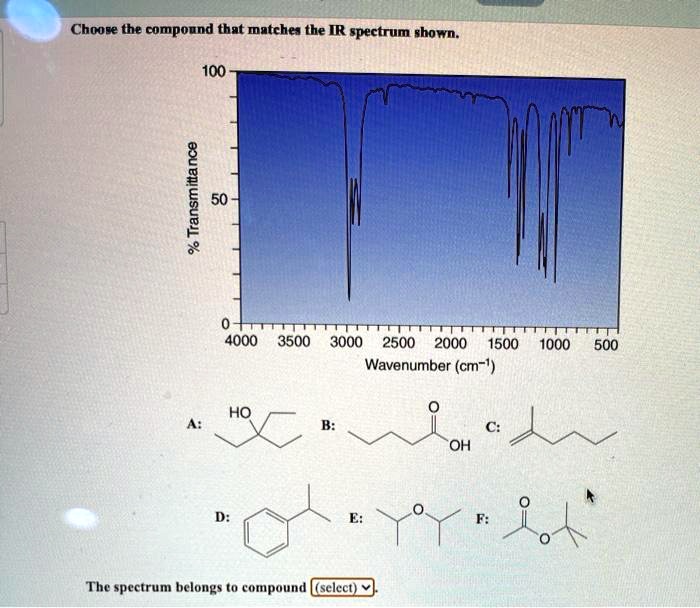
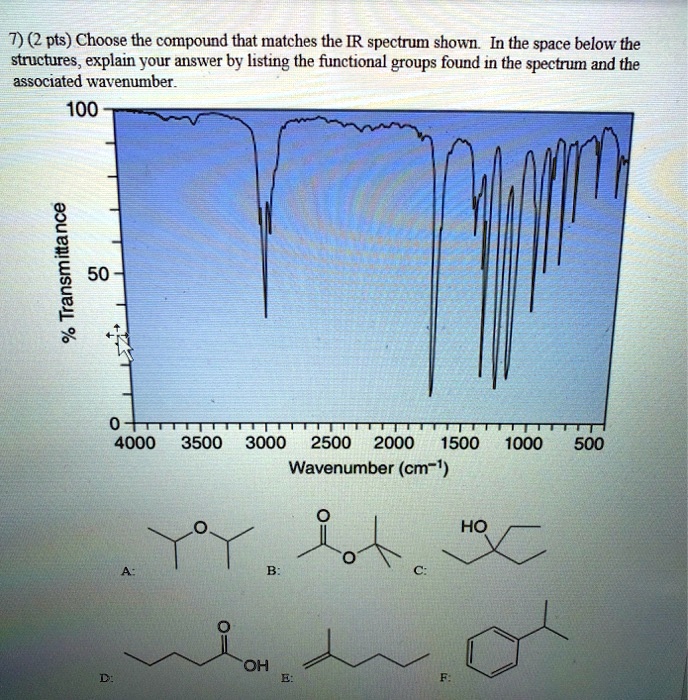
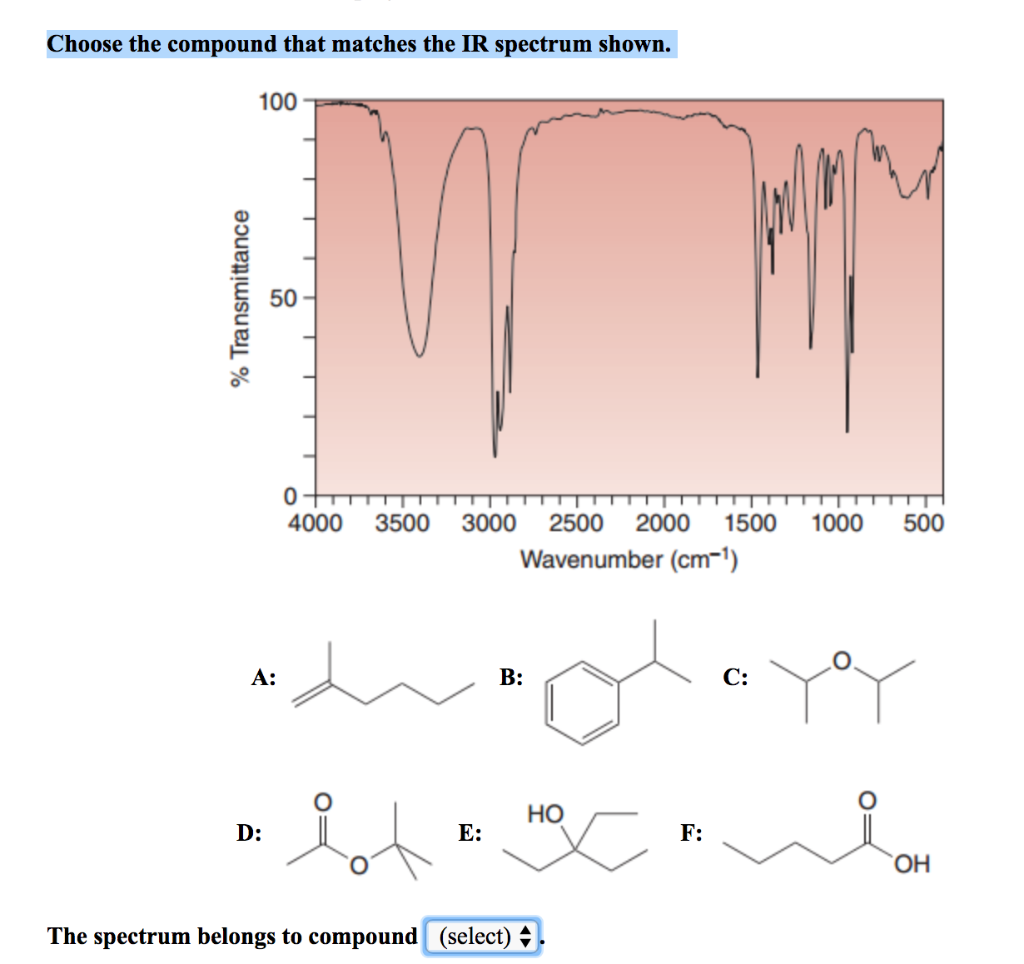
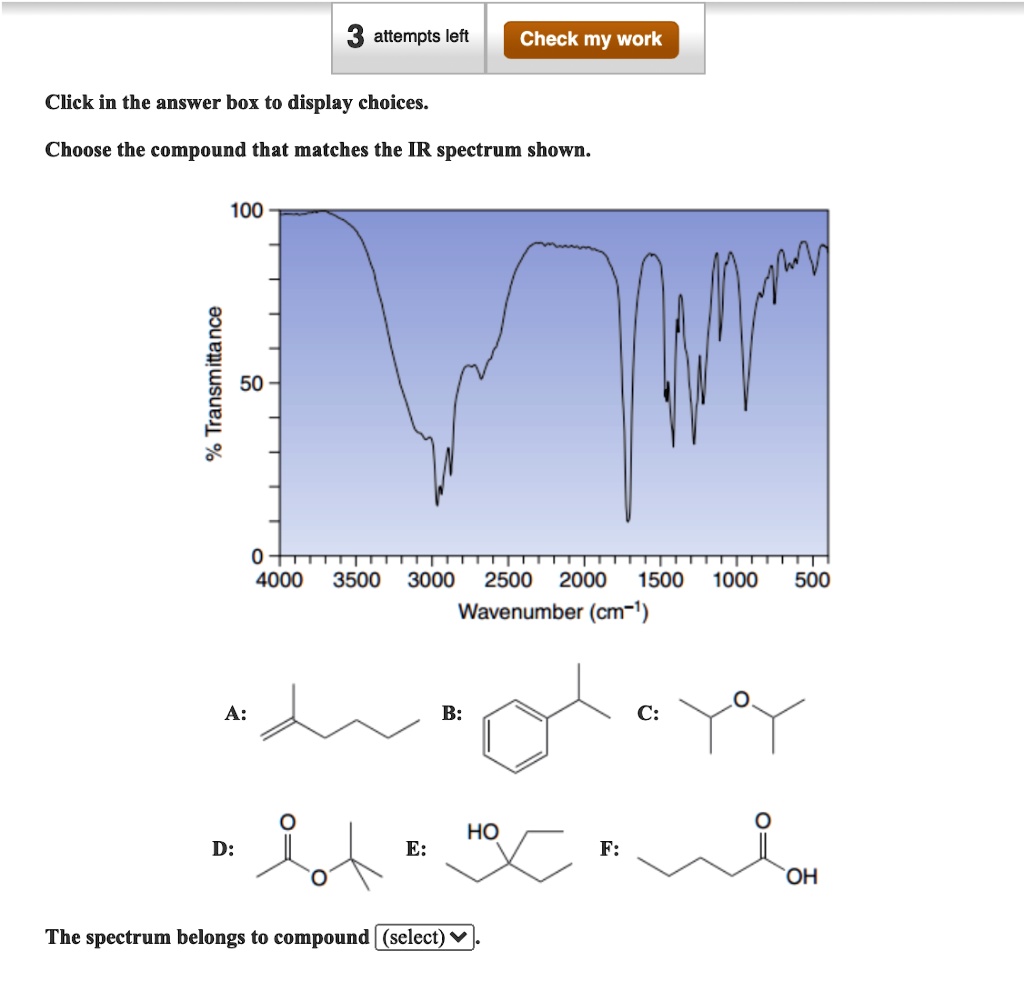
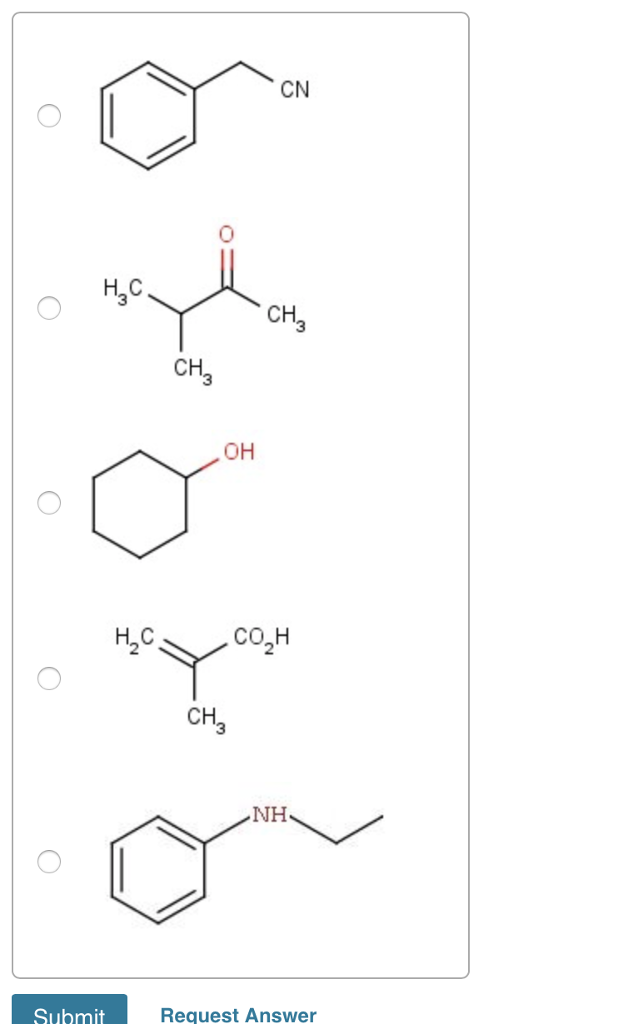
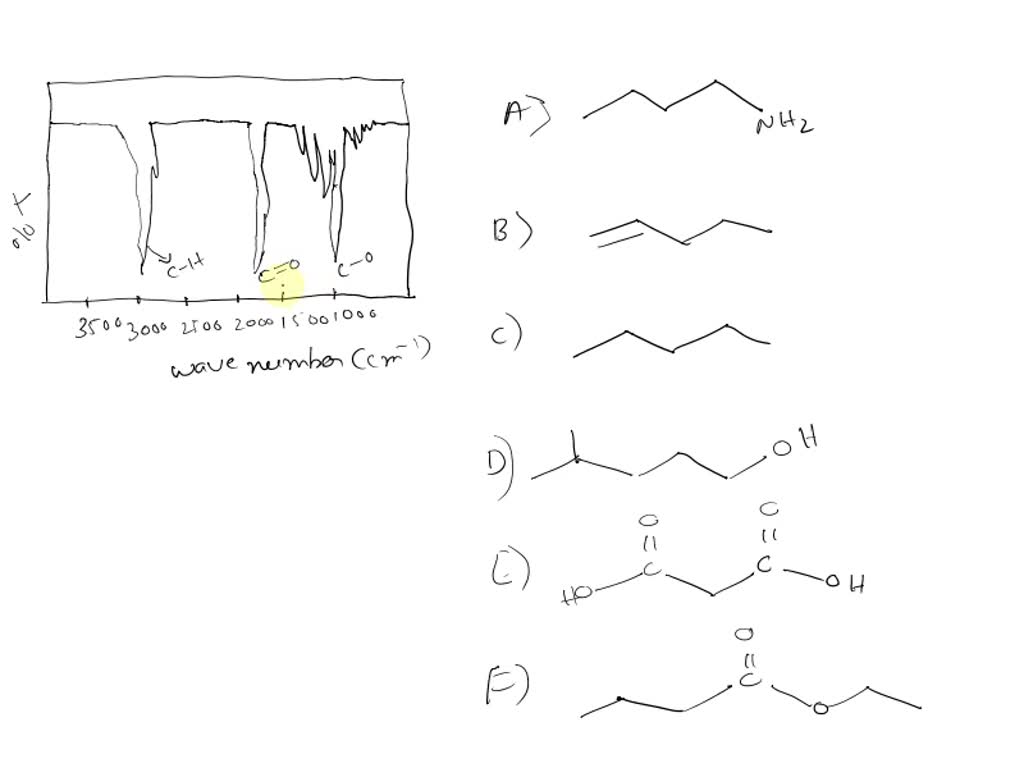
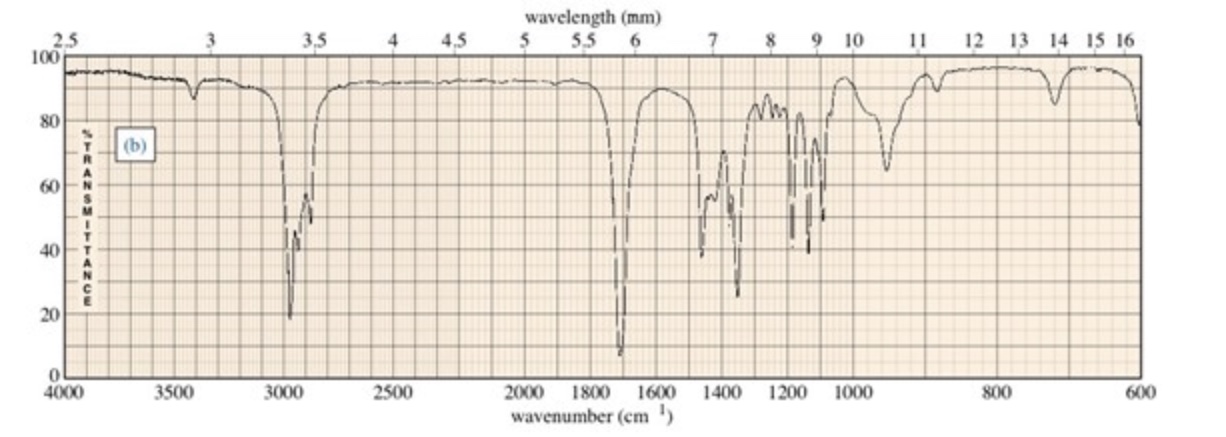
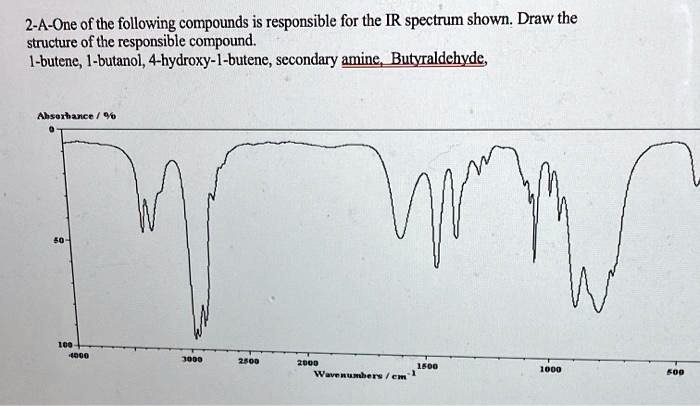
Choose The Compound Responsible For The Ir Spectrum Shown

A silent mystery, veiled in the language of spectral peaks, has gripped a team of analytical chemists. Their task: to definitively identify the organic compound responsible for a complex Infrared (IR) spectrum. The implications extend beyond academic curiosity, potentially impacting fields ranging from pharmaceutical quality control to environmental monitoring.
At the heart of this challenge lies the interpretation of vibrational fingerprints. These fingerprints uniquely characterize molecules based on how they absorb infrared radiation. The correct identification hinges on meticulous analysis and a deep understanding of molecular structure and bonding.
The spectrum in question presents a series of characteristic absorption bands. These bands, when deciphered, will point definitively to the compound's identity.
The Spectral Evidence: A Close Examination
The provided IR spectrum displays several key features. These features provide valuable clues, each absorption band corresponding to a specific vibrational mode within the molecule.
A strong, broad absorption around 3300 cm-1 immediately suggests the presence of an O-H stretch. Such a stretch indicates an alcohol or a carboxylic acid functional group is likely present.
A sharp peak near 1700 cm-1 often signifies a C=O stretch, a telltale sign of a carbonyl compound like a ketone, aldehyde, ester, or carboxylic acid.
The presence of absorptions in the 2850-3000 cm-1 region suggests C-H stretches. These stretches are commonly found in aliphatic compounds.
Dissecting the Possibilities
Given these initial observations, several candidate compounds emerge. Alcohols, ketones, aldehydes, esters, and carboxylic acids are all possibilities. However, distinguishing between these requires careful consideration of other spectral details.
For example, a carboxylic acid would typically show a very broad O-H stretch extending to lower wavenumbers. Additionally, its C=O stretch would appear at a slightly lower wavenumber than that of a ketone.
Esters would also exhibit a C=O stretch, but their spectra typically feature strong C-O stretches in the 1000-1300 cm-1 region. Ketones and aldehydes are differentiated by the presence of a distinctive C-H stretch for aldehydes around 2700-2800 cm-1.
Ruling Out and Narrowing Down
The absence of a very broad O-H stretch argues against a carboxylic acid. If the spectrum lacks the strong C-O stretches characteristic of esters, that class of compounds can also be tentatively ruled out.
Careful examination of the 2700-2800 cm-1 region is crucial. It helps determining whether an aldehyde is present.
If no aldehyde C-H stretch is visible, a ketone or alcohol remain the most likely candidates. Further differentiation requires analyzing subtle variations in the positions and intensities of other absorption bands.
Considerations Beyond the Spectrum
While the IR spectrum provides a wealth of information, it's rarely the sole piece of evidence used for compound identification. Supplementary data, such as Nuclear Magnetic Resonance (NMR) spectroscopy and mass spectrometry, offer complementary insights.
NMR spectroscopy provides information about the connectivity and environment of hydrogen and carbon atoms within the molecule. Mass spectrometry determines the molecular weight and fragmentation pattern, providing further clues about the compound's structure.
Combining these techniques provides a more robust and reliable identification than relying solely on IR spectroscopy.
Database Matching and Computational Analysis
Modern analytical chemistry often incorporates database searching. This involves comparing the unknown spectrum against libraries of known compounds. This provides a list of potential matches.
Computational methods can also be employed to predict the IR spectrum of candidate compounds. These predicted spectra can be directly compared to the experimental spectrum, providing another layer of validation.
These computational tools are particularly useful for complex molecules where spectral interpretation is challenging.
The Road Ahead: Achieving Certainty
Ultimately, identifying the compound responsible for the given IR spectrum requires a systematic and rigorous approach. Such approach integrates spectral analysis with complementary data and computational tools.
The chemist must carefully consider all available evidence. All available evidence has to be carefully considered before drawing a definitive conclusion.
Only then can the mystery of the unknown compound be solved. Only then can its role in wider scientific context be revealed, paving the way for advancements in various fields of research and industry.