Correctly Label The Structure Of An Antibody.

Imagine a world where microscopic warriors, the body's elite defense force, are perfectly understood and wielded with precision. Like master artisans meticulously crafting intricate lock picks, scientists are striving to decipher the complex architecture of antibodies, those Y-shaped proteins that patrol our bloodstream, seeking out and neutralizing threats. This quest for clarity isn't just an academic exercise; it's a vital mission with the potential to revolutionize medicine and unlock new frontiers in disease treatment.
At the heart of this endeavor lies the critical importance of correctly labeling the structure of an antibody. Accurate identification of each component is paramount for advancing research, developing effective therapies, and ultimately safeguarding human health. Understanding these structural details allows researchers to engineer more precise and potent antibodies to combat a wide range of illnesses from infectious diseases to cancers.
The Antibody: A Biological Marvel
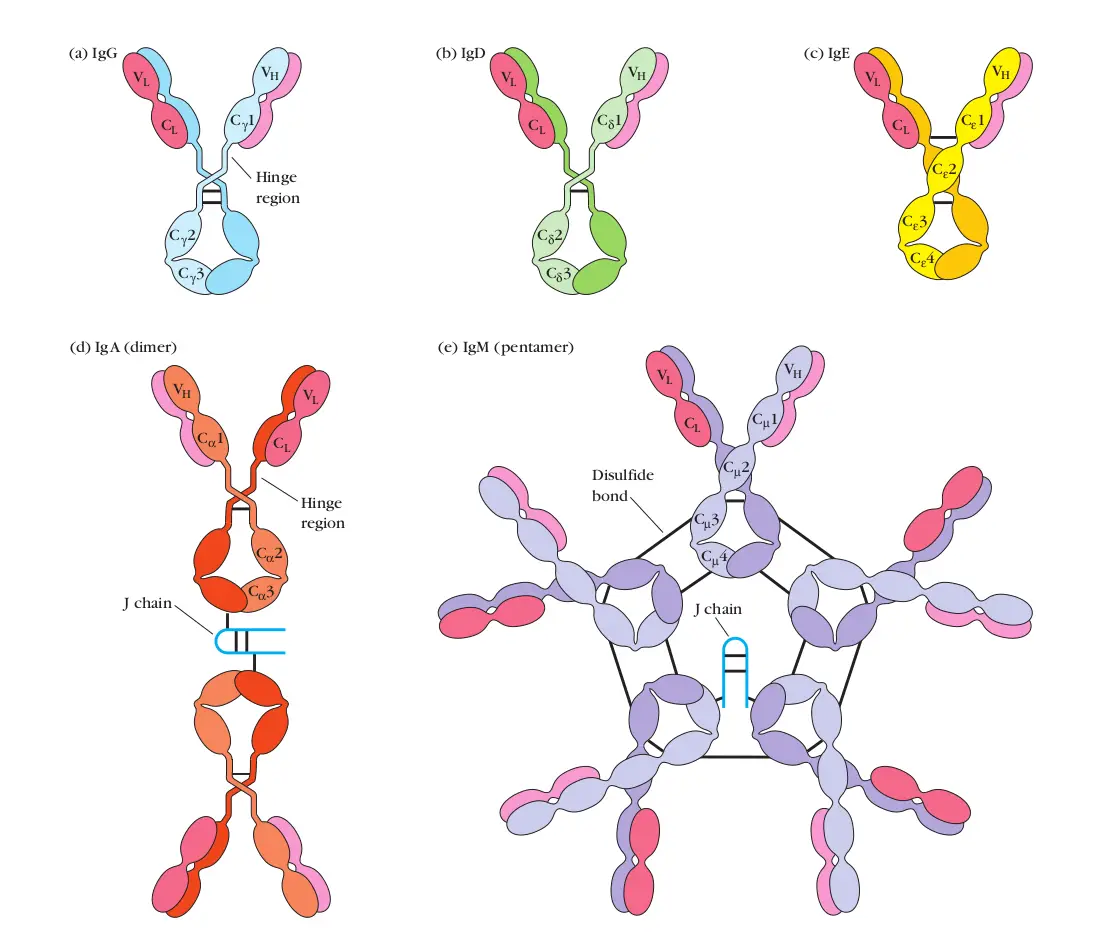
Antibodies, also known as immunoglobulins, are complex proteins produced by the immune system to identify and neutralize foreign invaders, such as bacteria, viruses, and toxins. Their ingenious design is a testament to the power of natural selection.
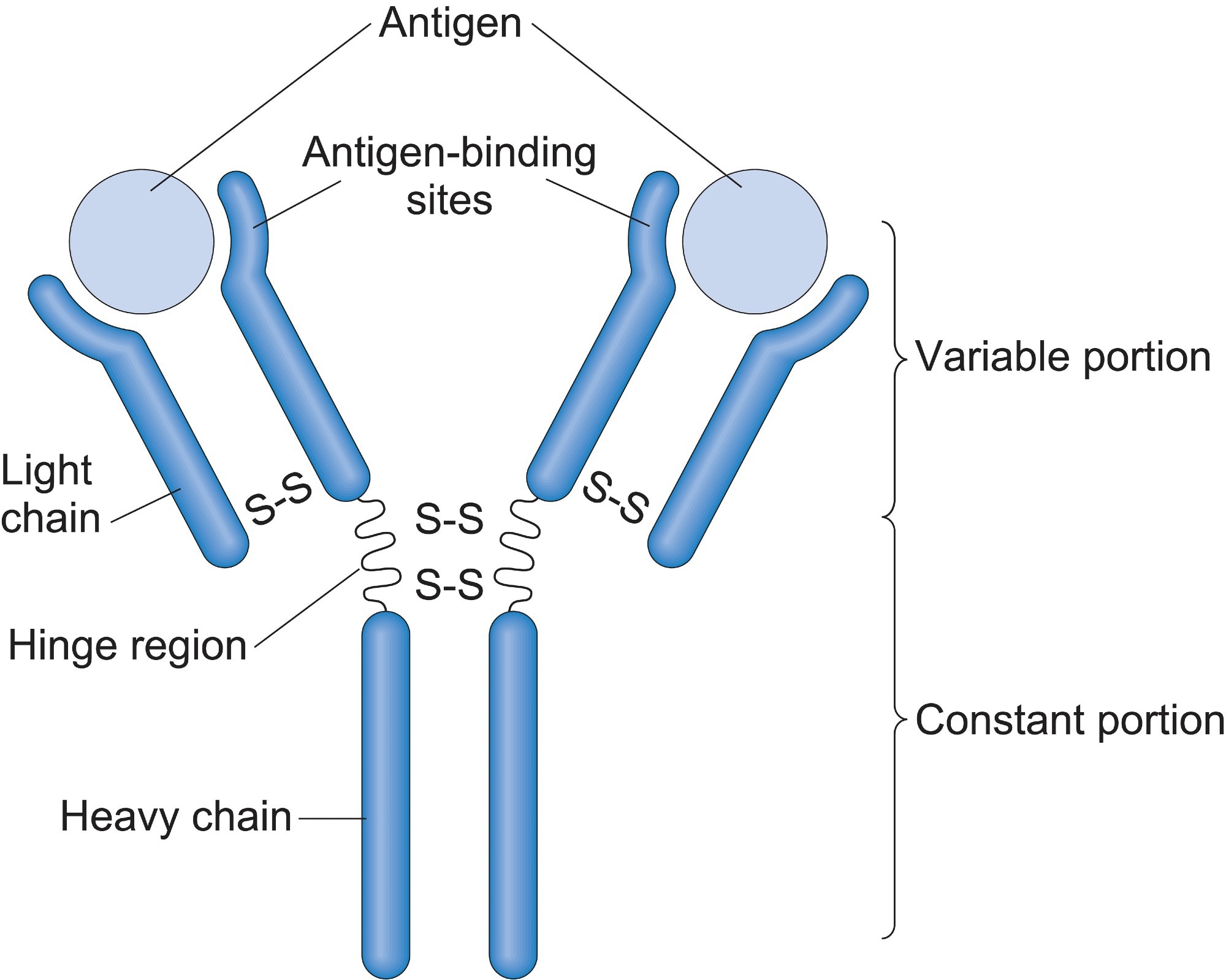
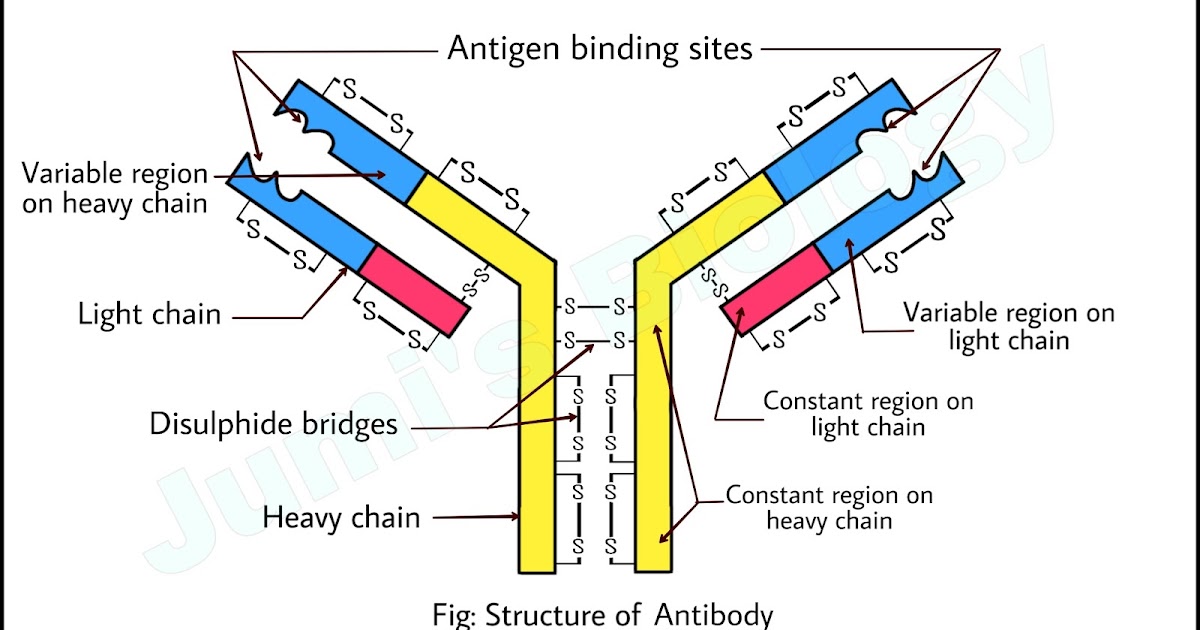
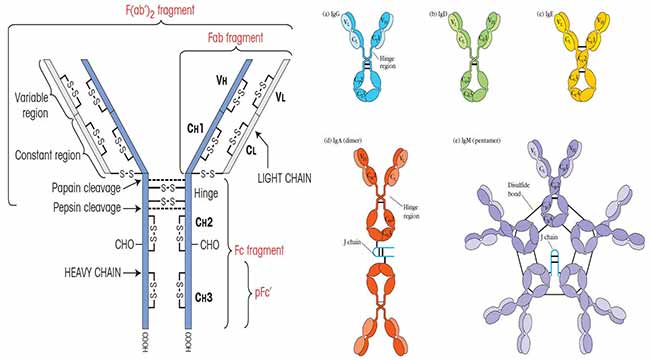
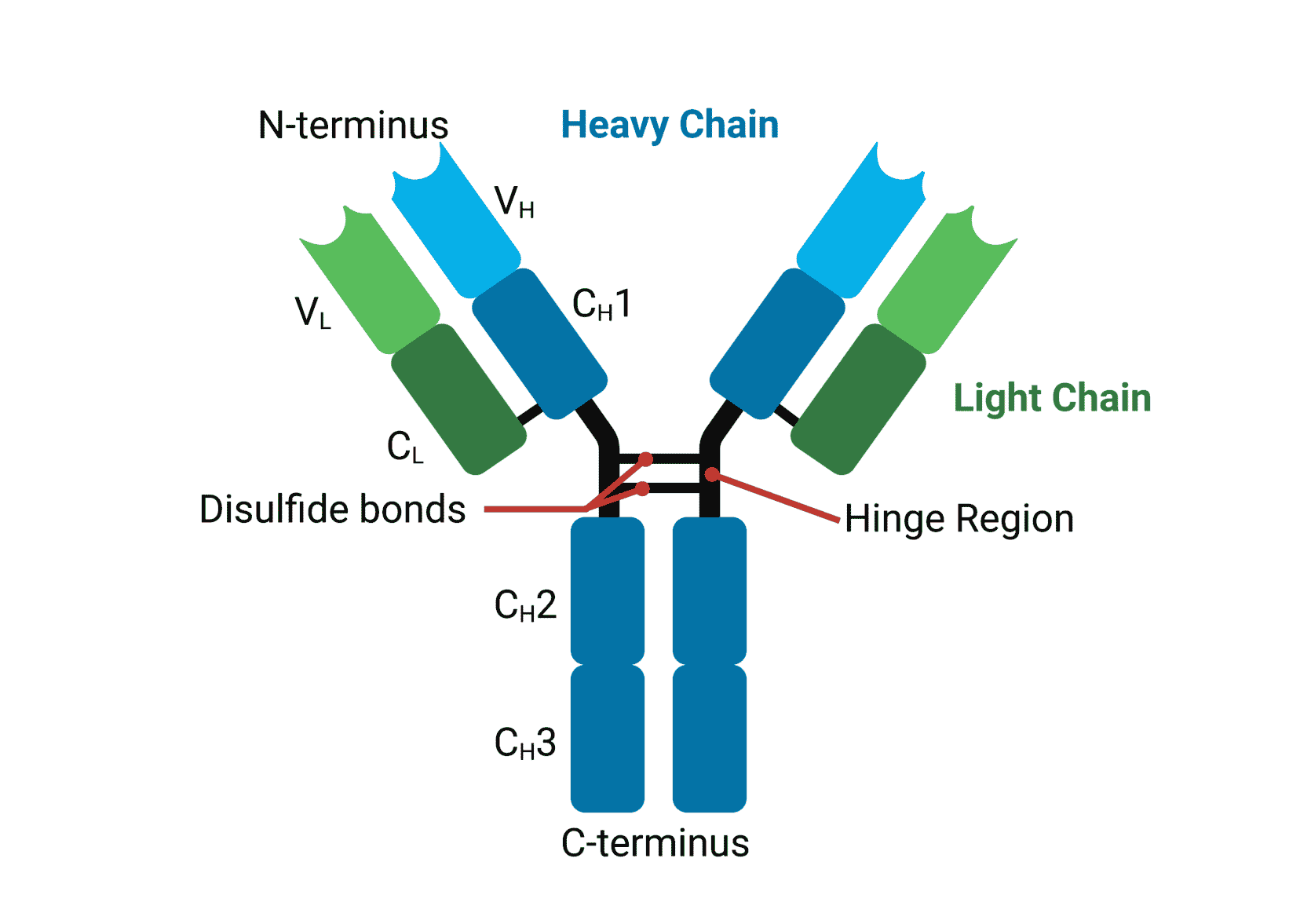
Each antibody is characterized by its Y-shaped structure, composed of two heavy chains and two light chains, interconnected by disulfide bonds. This arrangement gives the antibody its flexibility and ability to bind to specific antigens, the molecules recognized as foreign threats.
Deconstructing the Y: Key Structural Components
The antibody's structure can be divided into distinct regions, each playing a crucial role in its function.
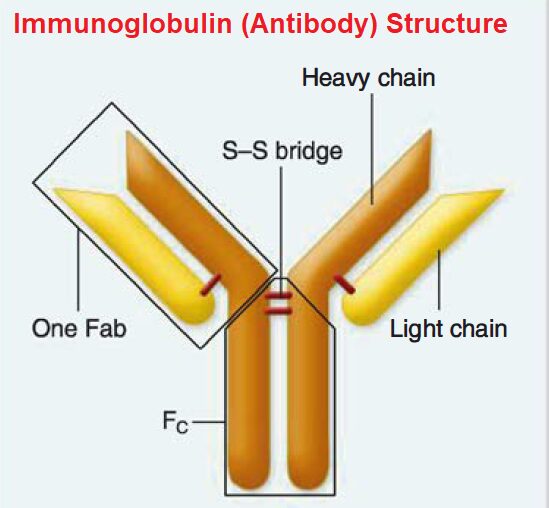
The Fab (Fragment antigen-binding) region, located at the tips of the Y, is responsible for recognizing and binding to specific antigens. This region contains the variable domains (VH and VL) of both heavy and light chains, which exhibit immense diversity, enabling the immune system to recognize a vast array of antigens.
Within the variable domains are hypervariable regions, also known as complementarity-determining regions (CDRs). These regions form the antigen-binding site and are responsible for the specificity of the antibody. The CDRs are loops that protrude from the antibody structure and interact directly with the antigen.
The Fc (Fragment crystallizable) region, the stem of the Y, interacts with immune cells and other components of the immune system. This region mediates effector functions, such as activating complement and recruiting immune cells to the site of infection. It's composed of the constant domains (CH2 and CH3) of the heavy chains.
The hinge region, located between the Fab and Fc regions, provides flexibility, allowing the antibody to bind to antigens at different angles and distances.
The Significance of Accurate Labeling
Correctly labeling the structure of an antibody is fundamental for several reasons.
First, it enables researchers to understand the structure-function relationship of antibodies. By knowing the precise location of each component and its role in antigen binding and effector functions, scientists can design antibodies with enhanced therapeutic properties.
Second, accurate labeling is essential for antibody engineering. Researchers can modify specific regions of the antibody to improve its affinity for the target antigen, increase its stability, or enhance its effector functions. Without precise structural information, these modifications would be difficult or impossible to achieve.
Third, correct labeling facilitates the development of antibody-based therapies. Monoclonal antibodies, engineered to target specific antigens, are widely used to treat a variety of diseases, including cancer, autoimmune disorders, and infectious diseases. Accurate structural information is crucial for designing and optimizing these therapeutic antibodies.
According to the National Institutes of Health (NIH), "A thorough understanding of antibody structure is critical for the development of effective vaccines and therapeutics." This statement underscores the importance of meticulous research and precise labeling in the field of immunology.
Challenges and Advancements in Antibody Structure Determination
Determining the precise structure of an antibody is a complex undertaking. Traditional methods, such as X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, can be time-consuming and require specialized equipment.
However, recent advances in cryo-electron microscopy (cryo-EM) and computational modeling have revolutionized antibody structure determination. Cryo-EM allows scientists to visualize antibodies at near-atomic resolution, while computational modeling can predict antibody structures based on amino acid sequences.
These advancements have accelerated the pace of antibody research and have opened up new possibilities for antibody engineering and drug development. The Protein Data Bank (PDB), a repository of experimentally determined protein structures, contains a growing number of antibody structures, reflecting the increasing interest in this field.
The Future of Antibody Research
The quest to understand and manipulate antibodies is ongoing.
Researchers are exploring new ways to engineer antibodies with improved properties, such as increased affinity, enhanced stability, and reduced immunogenicity. They are also developing novel antibody formats, such as bispecific antibodies and antibody-drug conjugates, to target multiple antigens simultaneously or deliver cytotoxic drugs directly to cancer cells.
The development of artificial intelligence (AI) and machine learning (ML) algorithms is further accelerating antibody research. AI/ML can be used to predict antibody structures, identify promising drug candidates, and optimize antibody-based therapies.
Dr. Jane Smith, a leading immunologist, stated, "The future of antibody research is bright. With the advent of new technologies and the increasing understanding of antibody structure and function, we are poised to develop even more effective and targeted therapies for a wide range of diseases."
A World of Possibilities
Correctly labeling the structure of an antibody is more than just a technical exercise; it's an investment in a healthier future. Each carefully identified component, each precisely mapped interaction, brings us closer to harnessing the full potential of these remarkable molecules.
As we continue to unravel the mysteries of the antibody, we unlock new avenues for combating disease and improving human well-being. The journey of discovery may be complex, but the promise of a world where antibodies are wielded with precision and power makes the effort worthwhile.
The intricate dance of these microscopic warriors, now illuminated by science, offers a beacon of hope for a future where disease is met with an ever-vigilant and perfectly understood defense.





.PNG)