Correctly Order The Steps Involved Cellular Immunity
The human body's ability to defend itself against a constant barrage of pathogens, from common viruses to deadly bacteria, hinges on a complex and precisely orchestrated immune response. A cornerstone of this defense is cellular immunity, a process relying on specialized cells to identify and eliminate infected or cancerous cells. Understanding the precise sequence of events in cellular immunity is crucial for developing effective vaccines and immunotherapies that can harness the body's natural defenses to combat disease.
At its core, cellular immunity involves a cascade of events triggered by the recognition of foreign antigens by immune cells, leading to the activation and proliferation of cytotoxic T lymphocytes (CTLs) and other immune cells. This process culminates in the targeted destruction of infected cells and the establishment of long-term immunity. This article will delve into the meticulously ordered steps of cellular immunity, drawing on current research and established immunological principles to clarify the sequence and significance of each phase. Accurate understanding is essential for advancements in disease treatment and prevention.
Antigen Presentation: The Trigger
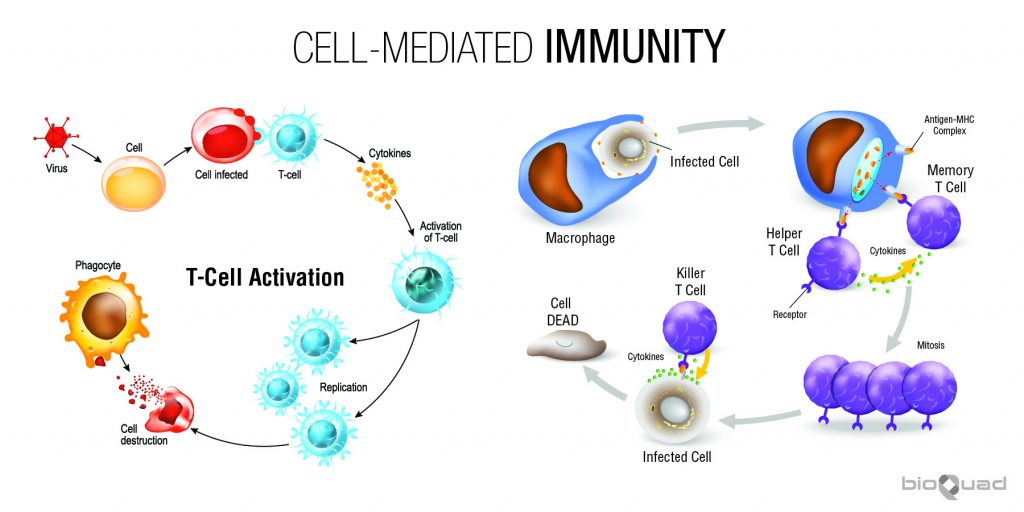
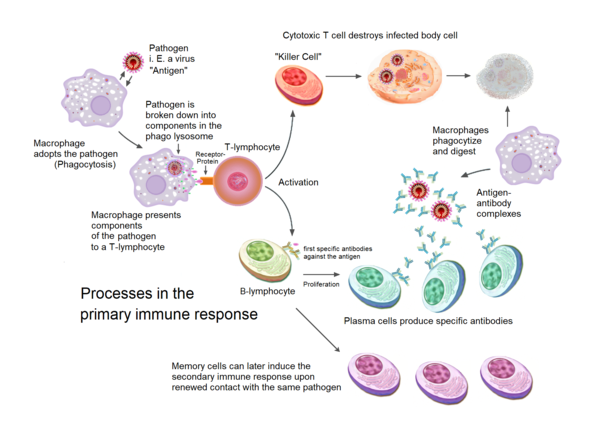
The journey of cellular immunity begins with antigen presentation. This critical step involves specialized cells, primarily antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells, capturing and processing foreign antigens.
These antigens, derived from pathogens or abnormal cells, are broken down into smaller peptide fragments within the APCs. Subsequently, these peptide fragments are loaded onto major histocompatibility complex (MHC) molecules.
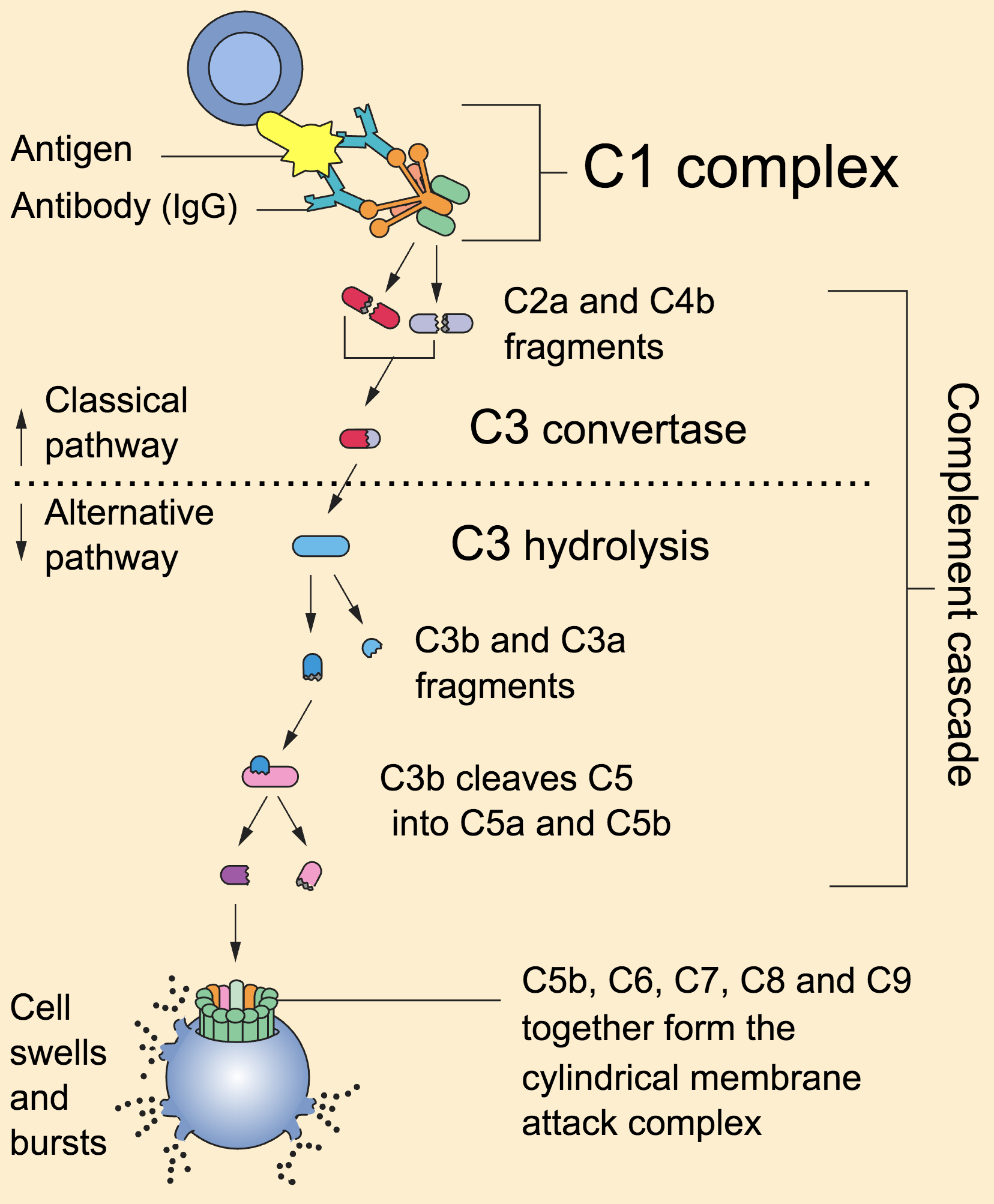
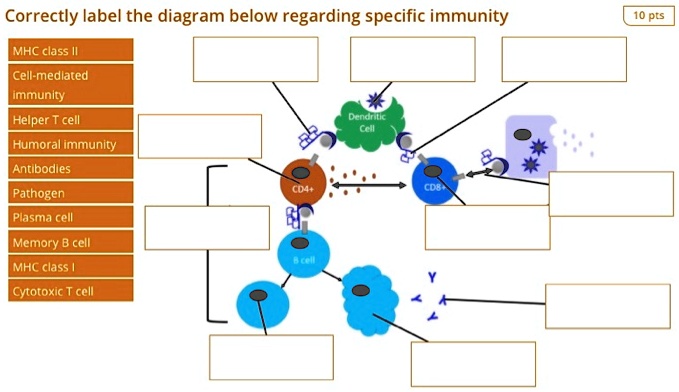
MHC molecules, found on the surface of APCs, act as signaling flags. There are two main classes: MHC class I, which presents antigens derived from the cell's interior (e.g., viral proteins), and MHC class II, which presents antigens acquired from the outside environment (e.g., bacterial antigens). The class dictates which type of T cell will be activated.
T Cell Activation: The Immune Command
Once the antigen-MHC complex is displayed on the APC surface, it's ready to interact with T cells. T cells are the foot soldiers of cellular immunity, and they possess T cell receptors (TCRs) that specifically recognize and bind to the antigen-MHC complex.
This interaction is not enough for full T cell activation. Co-stimulatory signals, delivered through interactions between molecules on the APC and the T cell (e.g., B7 on APCs binding to CD28 on T cells), are also required to prevent the T cell from becoming inactive or tolerized.
Only when both the TCR-MHC interaction and co-stimulation occur does the T cell become fully activated. This dual signal system ensures that T cells are activated only by genuine threats and not by self-antigens, preventing autoimmune reactions.
Clonal Expansion: Building the Army
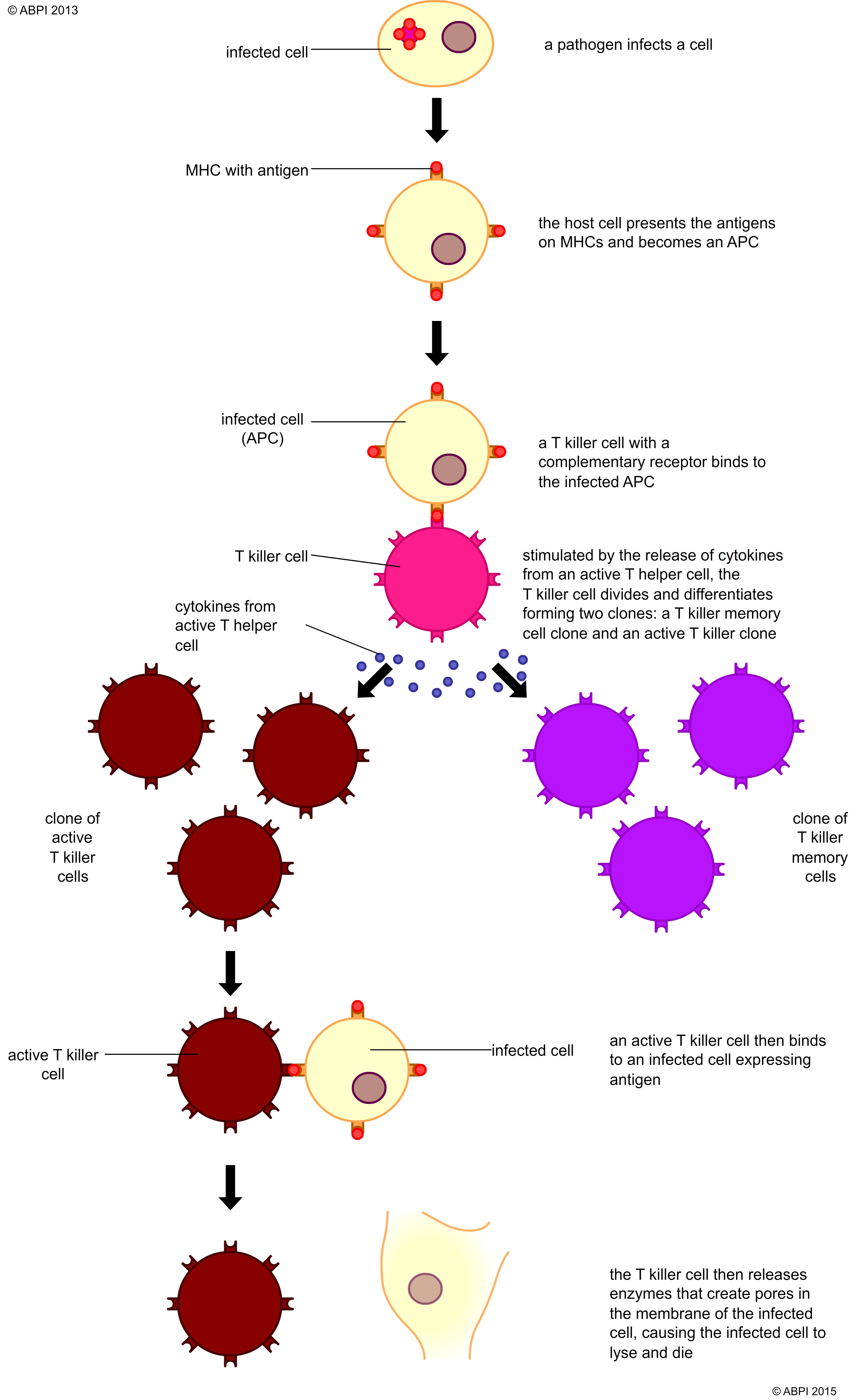
Upon activation, the T cell undergoes clonal expansion. This rapid proliferation creates a large population of T cells with the same TCR specificity, all capable of recognizing the same antigen.
This expansion is driven by the production of cytokines, signaling molecules that promote cell growth and division. Interleukin-2 (IL-2) is a key cytokine in this process, acting as a potent growth factor for T cells.
Clonal expansion is crucial for generating a sufficient number of effector cells to effectively combat the infection or eliminate cancerous cells. The magnitude of the expansion is tightly regulated to prevent excessive immune responses and tissue damage.
Differentiation: Specializing the Troops
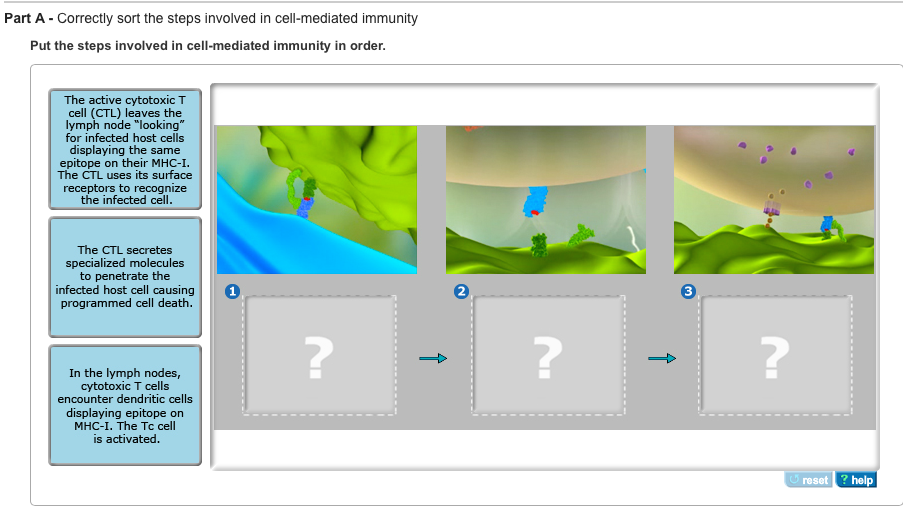
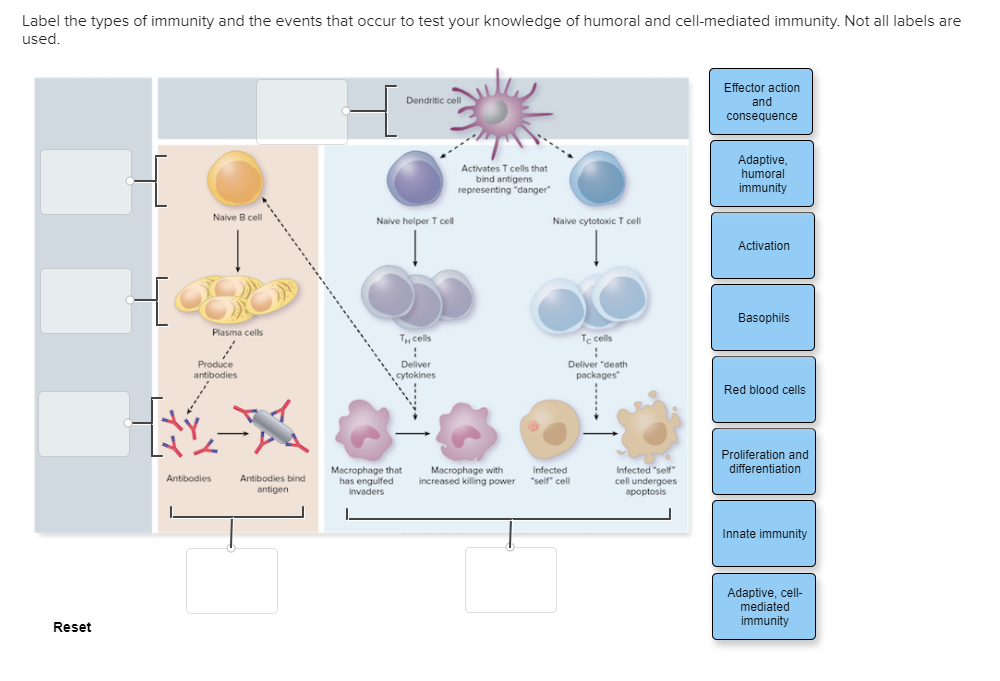
As T cells proliferate, they also differentiate into specialized subsets with distinct functions. For cellular immunity, the most important subset is the cytotoxic T lymphocyte (CTL), also known as CD8+ T cells.
CTLs are programmed to recognize and kill cells displaying the specific antigen that activated the original T cell. Other T cell subsets, such as helper T cells (CD4+ T cells), also play crucial roles in supporting CTL function by secreting cytokines that enhance their activity.
The differentiation process is driven by a complex interplay of cytokines and transcription factors, which ultimately determine the fate and function of the T cell.
Target Cell Recognition and Killing: The Attack
CTLs patrol the body, constantly scanning cells for signs of infection or abnormality. They achieve this by interacting with MHC class I molecules on the surface of all nucleated cells.
If a CTL encounters a cell displaying the target antigen-MHC class I complex, it binds to the target cell and initiates the killing process. This process involves the release of cytotoxic granules containing proteins such as perforin and granzymes.
Perforin creates pores in the target cell membrane, allowing granzymes to enter and activate caspases, a family of enzymes that trigger programmed cell death (apoptosis). Apoptosis ensures that the infected or cancerous cell is eliminated in a controlled manner, minimizing inflammation and damage to surrounding tissues.
Contraction and Memory: Remembering the Enemy
Once the infection is cleared or the cancerous cells are eliminated, the majority of effector T cells undergo apoptosis. This phase, known as contraction, is essential for restoring immune homeostasis and preventing chronic inflammation.
However, a small population of T cells survives and differentiates into memory T cells. These long-lived cells provide immunological memory, allowing the immune system to mount a faster and more robust response upon subsequent encounters with the same antigen.
Memory T cells can be further divided into different subsets with distinct characteristics and functions. Some reside in lymphoid organs, ready to rapidly activate upon antigen re-exposure, while others circulate throughout the body, providing frontline protection.
The Future of Cellular Immunity Research
A deeper understanding of the intricate steps involved in cellular immunity is paramount for developing more effective immunotherapies and vaccines. Researchers are actively exploring strategies to enhance antigen presentation, boost T cell activation, and improve the persistence and functionality of memory T cells.
Immunotherapies, such as checkpoint inhibitors and CAR T-cell therapy, are already revolutionizing cancer treatment by harnessing the power of cellular immunity. Further research is needed to overcome limitations and expand the applicability of these therapies.
Vaccines that effectively stimulate cellular immunity are crucial for preventing infectious diseases, particularly those caused by intracellular pathogens like viruses. Advances in vaccine design, such as the use of mRNA technology, hold great promise for eliciting potent and durable cellular immune responses. The journey to fully understanding and harnessing the power of cellular immunity is ongoing, but its potential to transform human health is undeniable.