Difference Between Electrolyte And Non Electrolyte
In the intricate dance of chemistry that sustains life, a fundamental distinction lies between substances that conduct electricity in solution – electrolytes – and those that do not – non-electrolytes. This seemingly simple difference has profound implications for biological processes, industrial applications, and even our everyday hydration strategies.
Understanding the disparity between electrolytes and non-electrolytes is crucial for comprehending how our bodies maintain fluid balance, nerve function, and muscle contractions. Moreover, in fields ranging from battery technology to water purification, this knowledge dictates the design and efficiency of various systems.
The Core Difference: Ionization
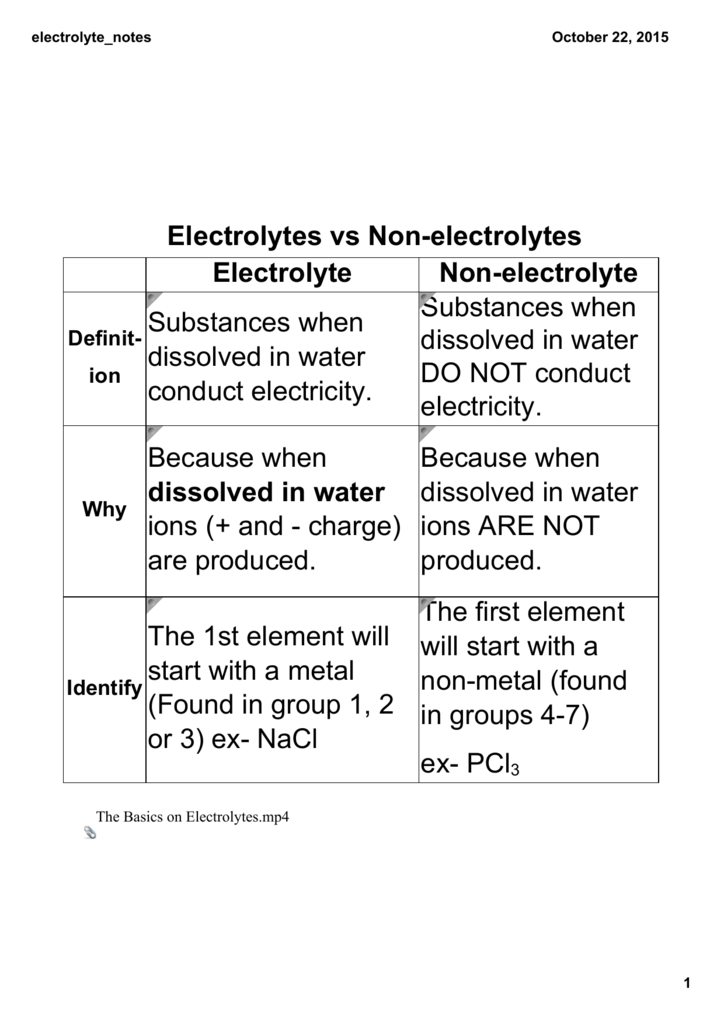
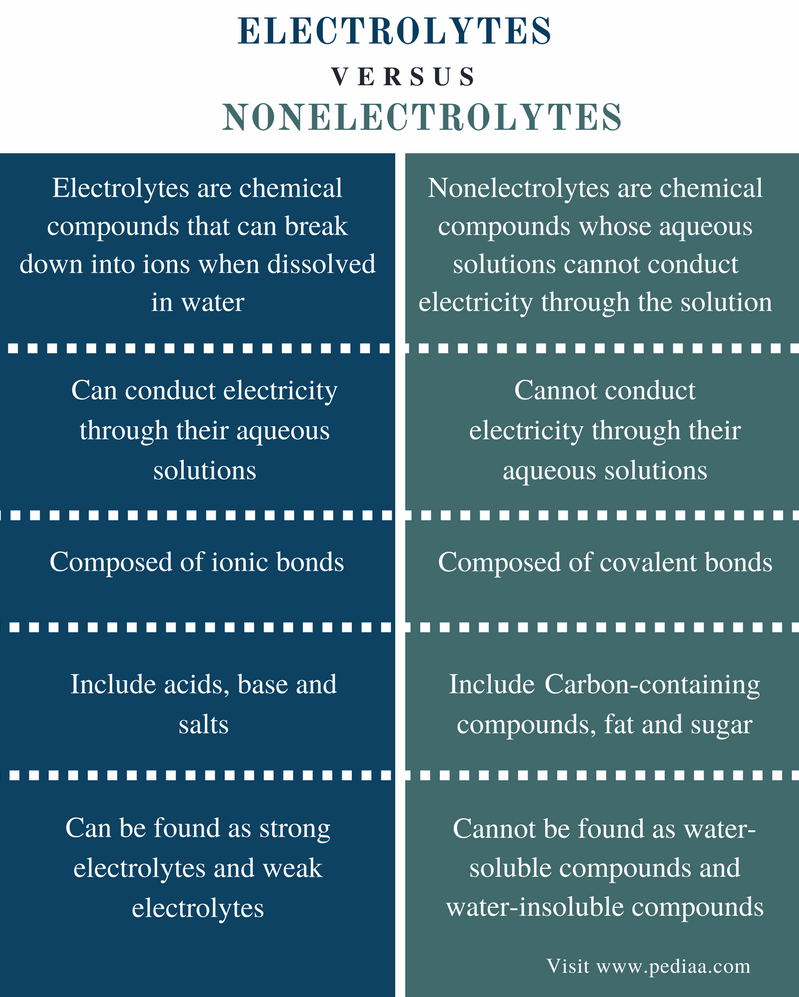
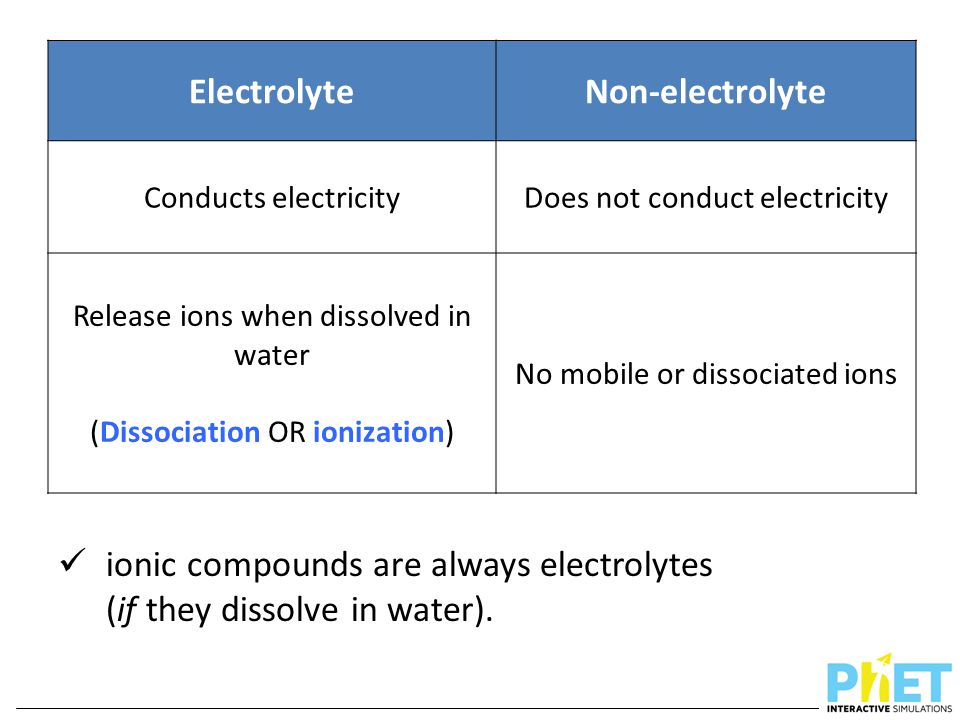
The key distinction boils down to a substance's ability to dissociate into ions when dissolved in a solvent, typically water. Electrolytes undergo ionization, meaning they break apart into positively charged cations and negatively charged anions.
These ions, freely moving within the solution, act as charge carriers, enabling the solution to conduct electricity. Non-electrolytes, on the other hand, do not dissociate into ions in solution.
Instead, they remain as neutral molecules, thus failing to facilitate electrical conductivity.
Electrolytes: Conductors of Life
Electrolytes are vital for numerous biological functions. These functions include maintaining osmotic pressure, nerve impulse transmission, and muscle contraction, electrolytes play a crucial role.
Common electrolytes in the human body include sodium (Na+), potassium (K+), chloride (Cl-), calcium (Ca2+), and magnesium (Mg2+). These are precisely regulated to maintain homeostasis.
Dehydration, excessive sweating, or certain medical conditions can lead to electrolyte imbalances, resulting in symptoms ranging from muscle cramps and fatigue to more severe complications like seizures and cardiac arrhythmias.
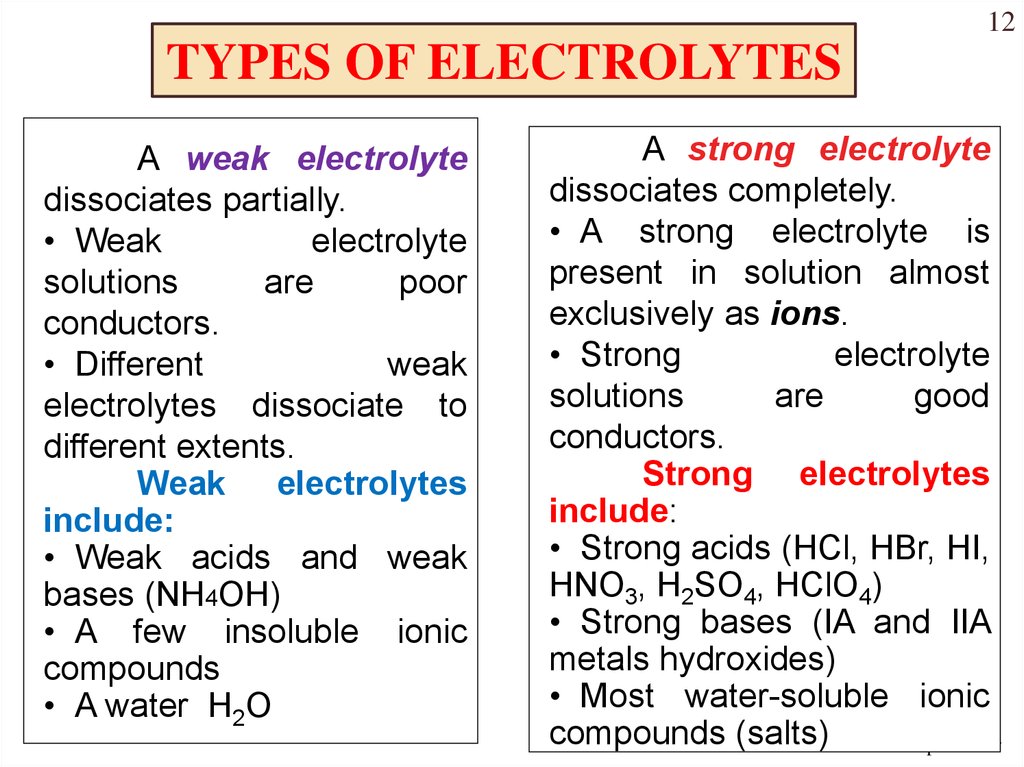
Types of Electrolytes: Strong vs. Weak
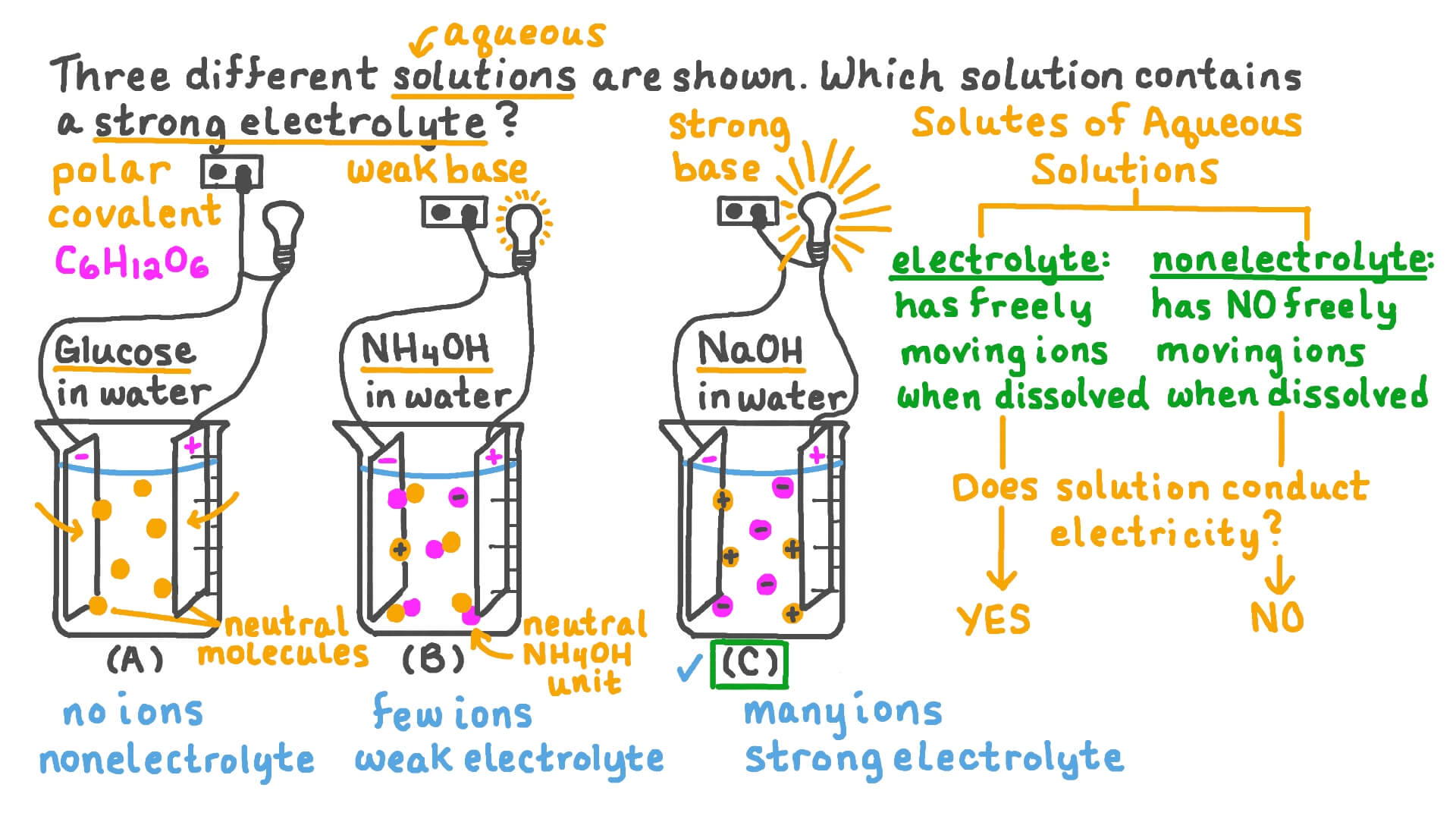
Electrolytes are further classified as strong electrolytes and weak electrolytes. Strong electrolytes completely dissociate into ions when dissolved in water.
Examples include sodium chloride (NaCl) and hydrochloric acid (HCl). Their solutions are highly conductive.
Weak electrolytes, conversely, only partially dissociate. Acetic acid (CH3COOH) and ammonia (NH3) are common examples, and their solutions exhibit lower conductivity.
The degree of dissociation is governed by the equilibrium constant of the ionization reaction, with strong electrolytes having much larger equilibrium constants than weak electrolytes.
Non-Electrolytes: Molecular Solutions
Non-electrolytes dissolve in water but do not form ions. Sugar (sucrose), ethanol, and urea are typical examples.
When these substances dissolve, their molecules disperse throughout the solvent without breaking apart into charged particles. Consequently, their solutions do not conduct electricity.
While non-electrolytes do not directly contribute to electrical conductivity, they can still play important roles in biological and industrial processes. For example, glucose provides energy to cells.
Applications of Non-Electrolytes
Non-electrolytes find use in various industrial processes. These range from creating solvents, to drug delivery system development. Also, they are used in pharmaceutical applications.
Their inertness in terms of electrical conductivity is often an advantage in these contexts, allowing for precise control over reaction conditions and solution properties.
In medicine, non-electrolyte solutions are used for intravenous administration of nutrients and medications, providing a safe and effective means of delivery without disrupting the body's electrolyte balance.
Distinguishing Electrolytes and Non-Electrolytes: Experimental Methods
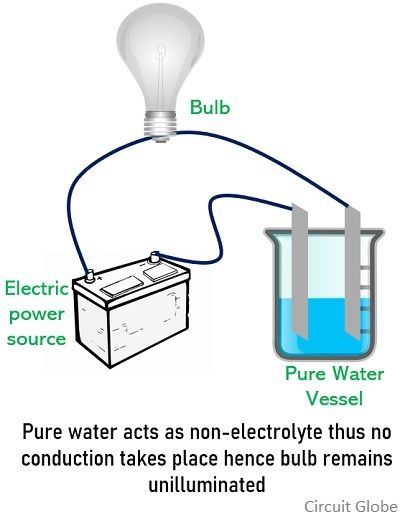
The most direct method for distinguishing between electrolytes and non-electrolytes is to measure the electrical conductivity of their solutions. A conductivity meter can accurately determine the solution's ability to conduct electricity.
High conductivity indicates the presence of ions and thus identifies the substance as an electrolyte. Conversely, low or negligible conductivity suggests a non-electrolyte.
Other methods include observing the colligative properties of solutions, such as boiling point elevation and freezing point depression. Electrolyte solutions exhibit greater changes in these properties compared to non-electrolyte solutions of the same concentration, due to the increased number of particles (ions) in solution.
Implications and Applications
The distinction between electrolytes and non-electrolytes has far-reaching implications across diverse fields. In medicine, understanding electrolyte balance is crucial for diagnosing and treating various conditions. This includes dehydration, kidney disease, and heart failure.
In sports science, athletes need to replenish electrolytes lost through sweat to maintain optimal performance and prevent muscle cramps. Sports drinks are formulated to provide a balance of electrolytes and carbohydrates for this purpose.
In industry, electrolytes are essential components of batteries, fuel cells, and electroplating processes. The efficiency and performance of these technologies depend on the properties of the electrolytes used.
"The proper balance of electrolytes is paramount for maintaining cellular function and overall health," notes Dr. Emily Carter, a leading biochemist at the National Institutes of Health.
Furthermore, electrolytes play a critical role in water purification and wastewater treatment. Electrolytic processes can be used to remove contaminants and disinfect water, providing safe and clean drinking water.
Future Directions
Research into new and improved electrolytes is ongoing. Scientists are seeking to develop electrolytes with higher conductivity, greater stability, and enhanced environmental friendliness.
One promising area is the development of solid-state electrolytes for batteries. This would enhance safety and energy density compared to liquid electrolytes.
Another area of focus is the design of novel electrolyte solutions for biomedical applications. These applications are more biocompatible and effective for drug delivery and tissue engineering.
Ultimately, a deeper understanding of the fundamental differences between electrolytes and non-electrolytes is crucial for advancing scientific knowledge and developing innovative technologies that benefit society.






.PNG)