Does Atp Or Adp Have Higher Potential Energy
The fundamental question of which molecule, Adenosine Triphosphate (ATP) or Adenosine Diphosphate (ADP), possesses higher potential energy is a cornerstone of understanding cellular energy dynamics. This seemingly simple query delves into the intricate mechanisms that power life, influencing fields from biochemistry to sports science.
At the heart of this discussion lies the concept of chemical potential energy, stored within the bonds of these molecules. Understanding their energy differences is crucial for comprehending how cells fuel vital processes. This knowledge impacts diverse areas like drug development and athletic performance optimization.
The Players: ATP and ADP
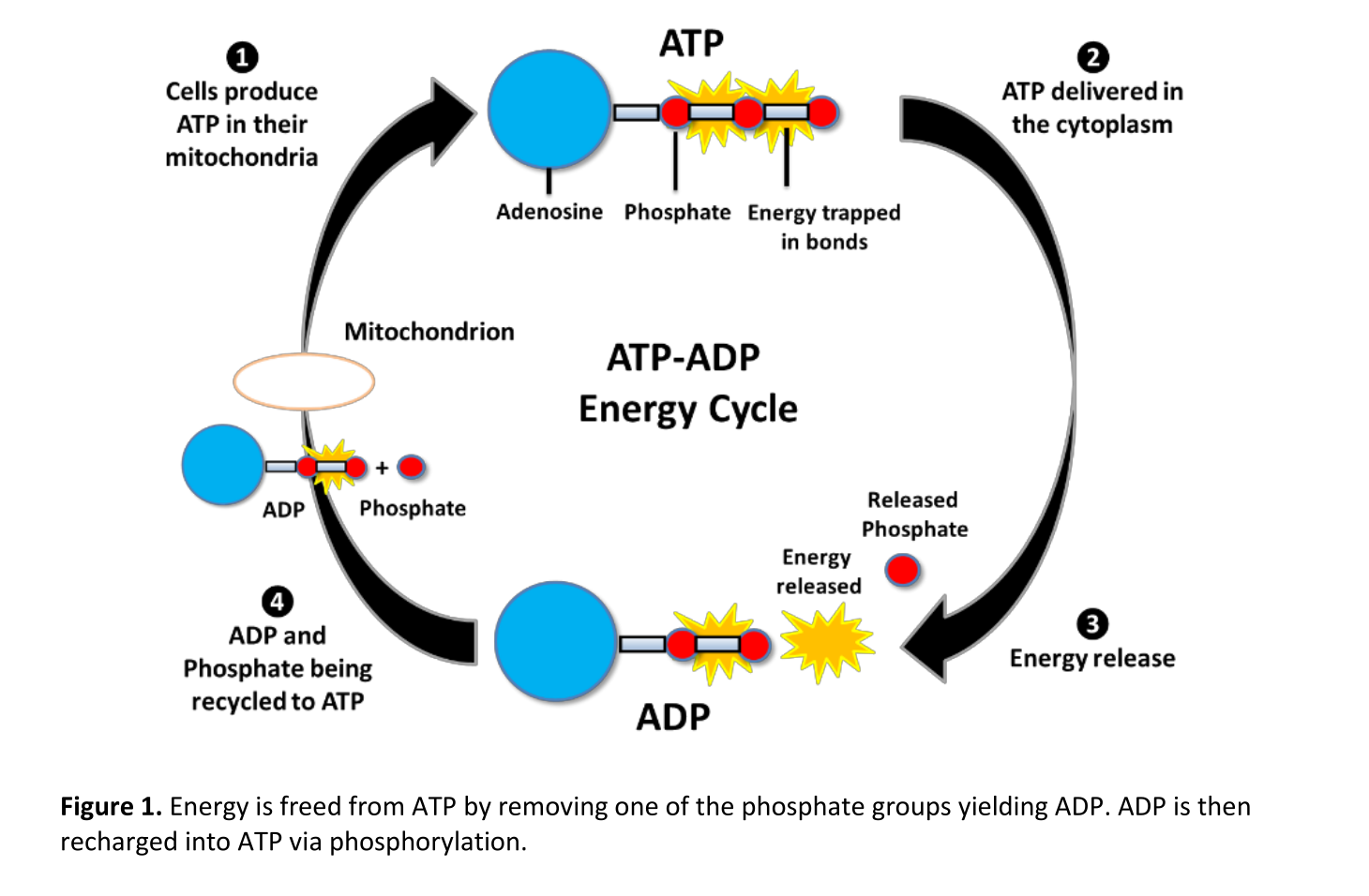
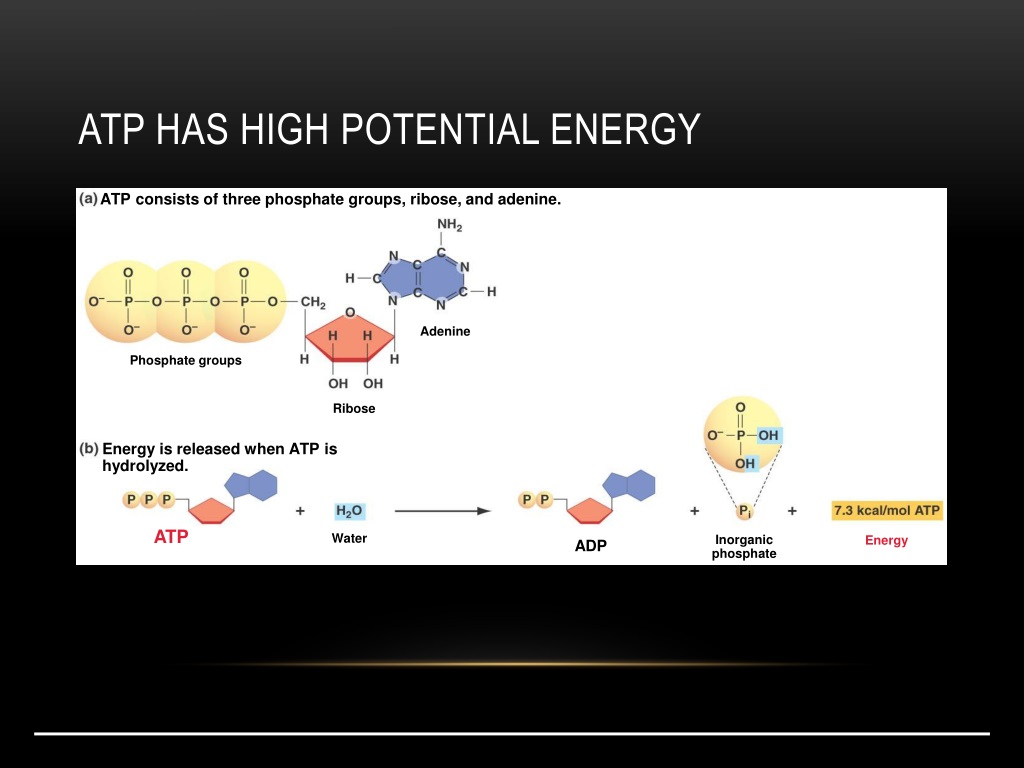
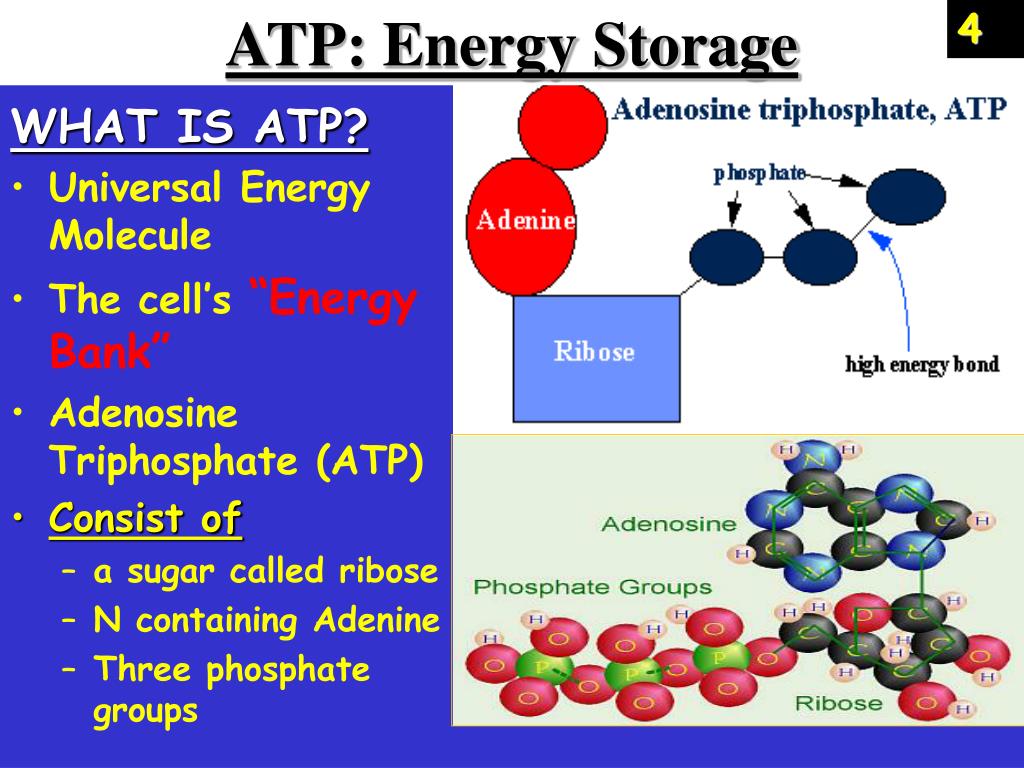
ATP, or Adenosine Triphosphate, is often called the "energy currency" of the cell. It consists of an adenosine molecule bonded to three phosphate groups. ADP, or Adenosine Diphosphate, is similar but contains only two phosphate groups.
The crucial difference lies in the number of phosphate groups. This seemingly small distinction leads to significant variations in energy storage capabilities.
Potential Energy: A Matter of Bonds
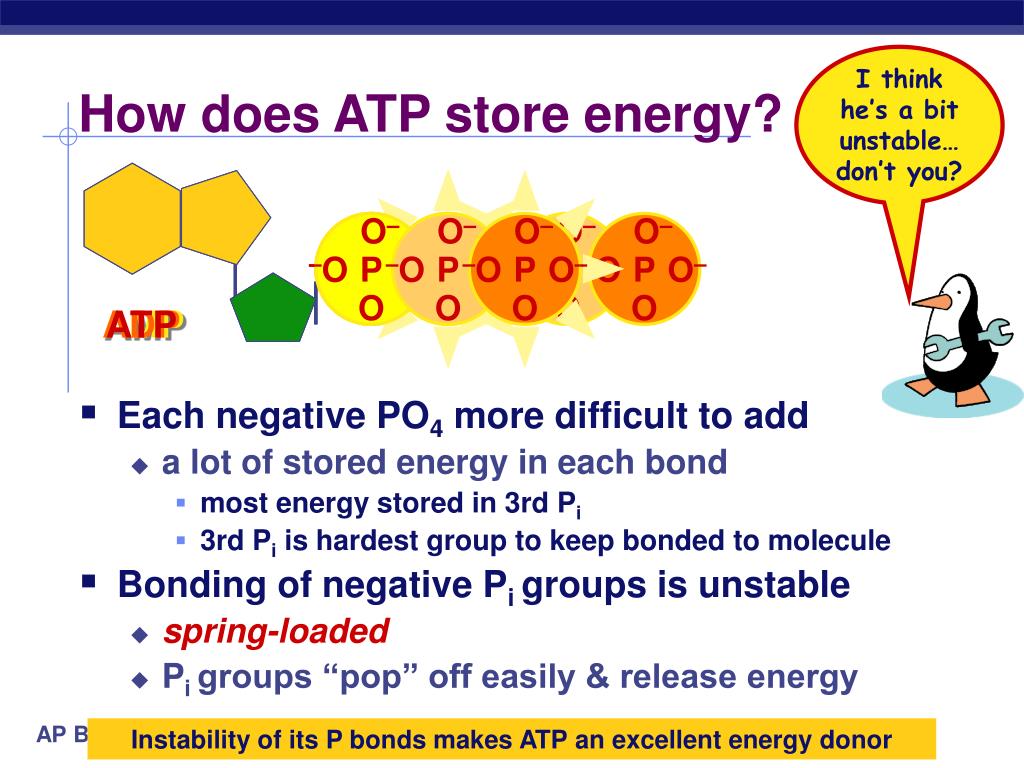
The potential energy within ATP and ADP is primarily stored in the phosphate bonds. These bonds, particularly the terminal phosphate bond in ATP, are high-energy bonds.
Breaking these bonds releases energy that the cell can then harness to perform work. This energy release drives a multitude of cellular processes.
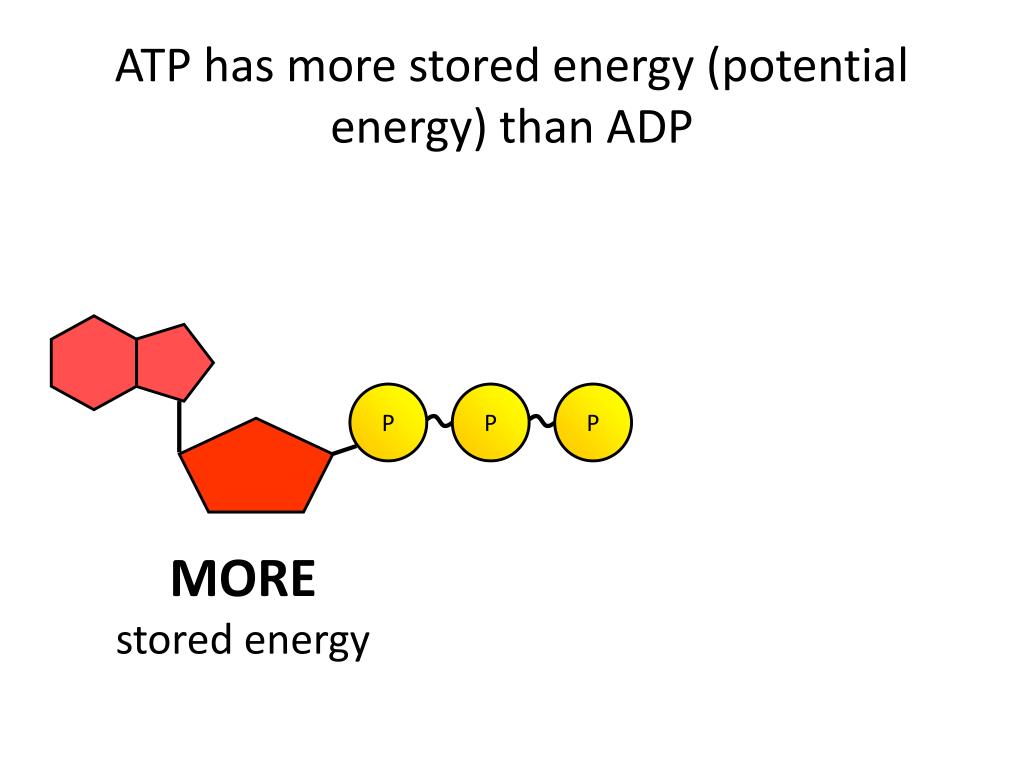
Why ATP Holds More Energy
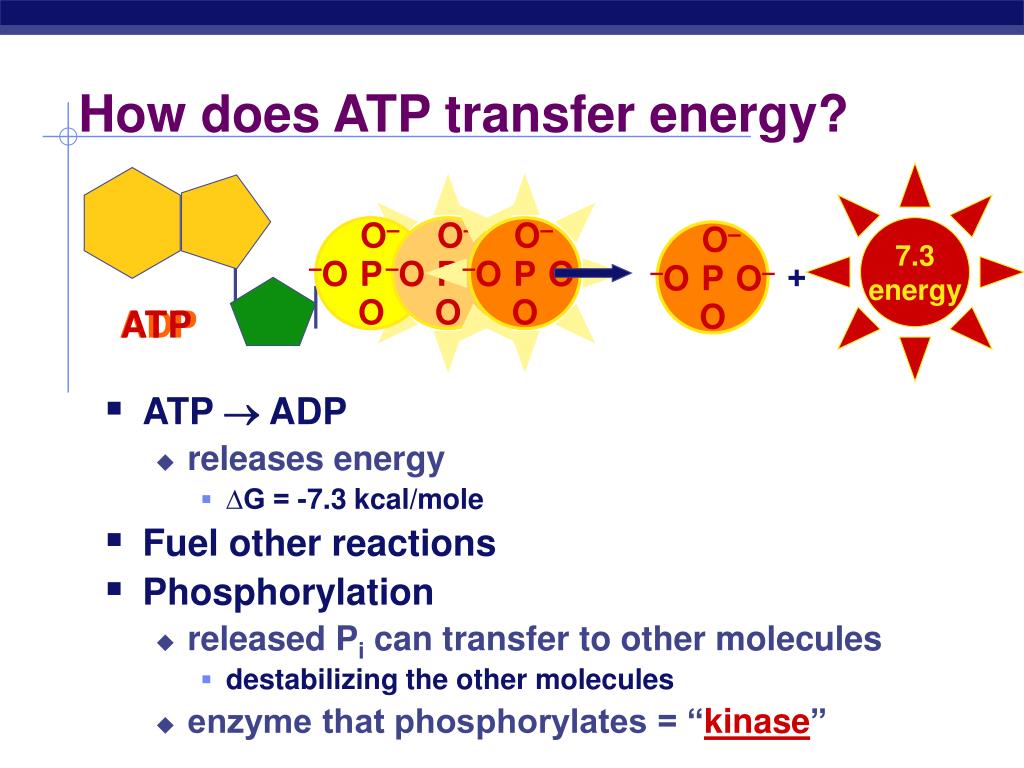
The key reason ATP possesses higher potential energy is the presence of that third phosphate group. The three negatively charged phosphate groups in ATP repel each other strongly.
This electrostatic repulsion creates a state of inherent instability. The bond connecting the terminal phosphate group is therefore strained, requiring considerable energy to maintain its integrity.
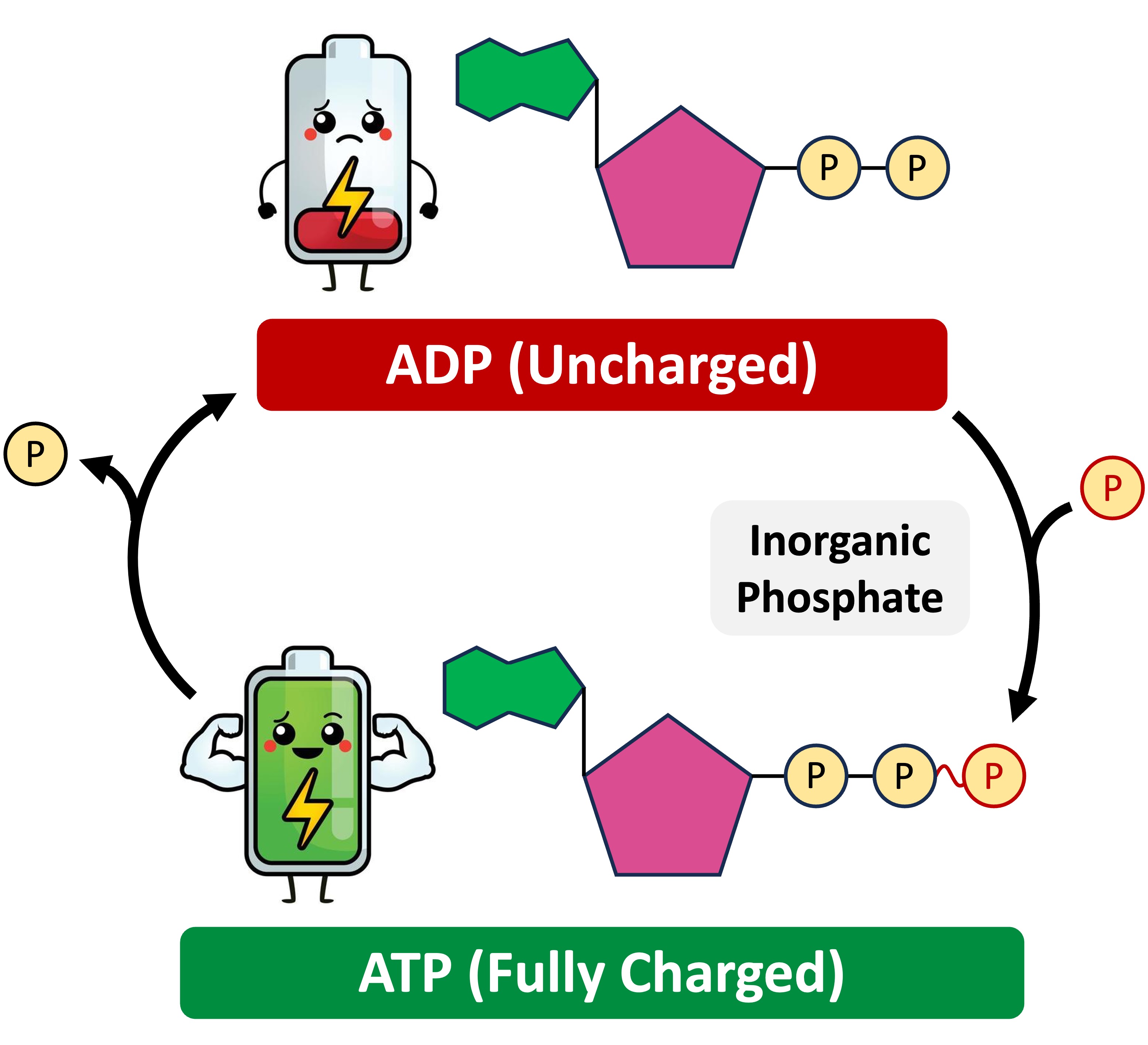
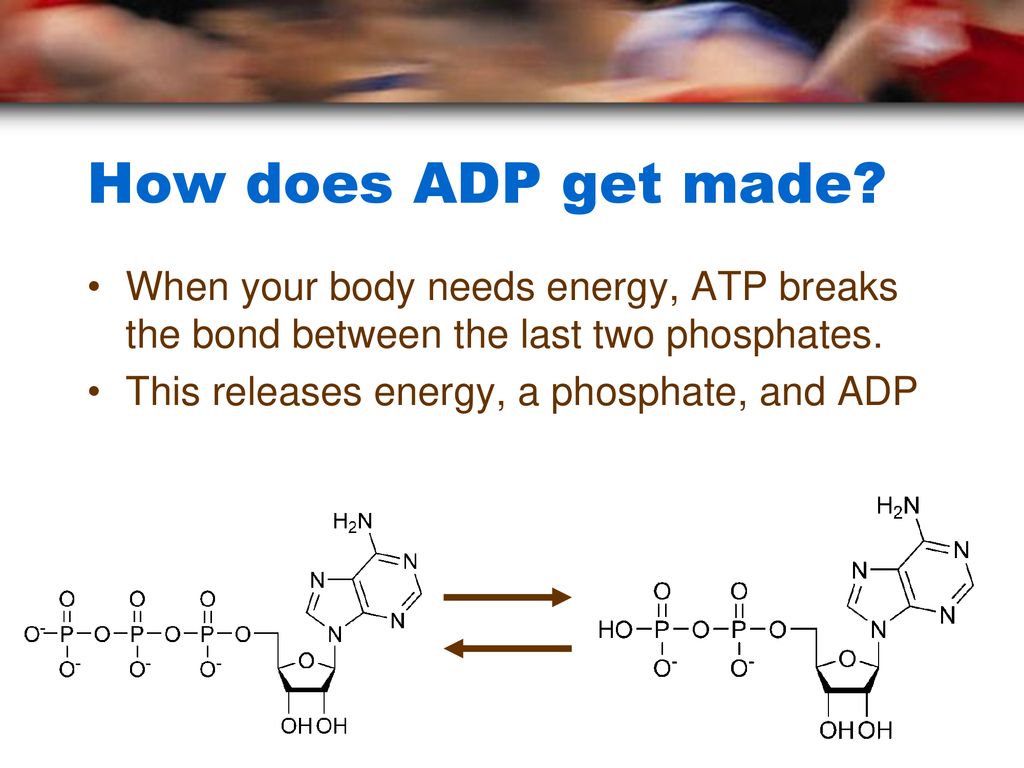
When this terminal phosphate bond is broken via hydrolysis, releasing inorganic phosphate (Pi), this stored potential energy is released. This transforms ATP into ADP.
The Hydrolysis Reaction: Energy Release
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. This released energy is then used to power endergonic reactions, which require energy input, within the cell.
Examples of endergonic processes fueled by ATP hydrolysis include muscle contraction, nerve impulse transmission, and protein synthesis.
"The hydrolysis of ATP to ADP is a fundamental process powering almost all cellular activities. Understanding this process is vital to many scientific fields," stated Dr. Eleanor Vance, a biochemist at the National Institutes of Health.
The conversion of ATP to ADP is a continuous cycle. ADP is then phosphorylated, adding a phosphate group back on, reforming ATP via cellular respiration or photosynthesis.
Impact on Biological Systems
The higher potential energy of ATP compared to ADP has profound implications for biological systems. It directly relates to how cells obtain and utilize energy.
For example, during intense exercise, muscle cells rapidly deplete ATP stores. The body quickly regenerates ATP from ADP to sustain muscle function. If ATP cannot be produced at the needed rate, fatigue sets in.
Medical and Research Applications
Understanding the ATP/ADP energy dynamic is crucial in medical research. Researchers are exploring ways to manipulate ATP production and consumption to treat diseases.
Certain drugs target ATP synthesis pathways in cancer cells, disrupting their energy supply and hindering growth. Similarly, therapies aimed at improving mitochondrial function (the site of ATP production) are being investigated for neurological disorders.
Furthermore, scientists use ATP as an energy source to power in vitro reactions. This is a common practice in many biochemical experiments.
Potential Societal Impact
The understanding of cellular energy, driven by the ATP/ADP cycle, has far-reaching societal implications. Developments in areas such as athletic performance enhancement and disease treatment are directly linked to this understanding.
Improved athletic performance, for instance, might be achieved through optimized ATP regeneration pathways. This is the goal of several sports nutrition companies.
Effective treatments for energy-related diseases like mitochondrial disorders could significantly improve the quality of life for affected individuals and their families.
Conclusion
In conclusion, ATP possesses higher potential energy than ADP due to the presence of the third phosphate group. The hydrolysis of ATP to ADP releases energy, fueling a vast array of cellular processes critical for life.
This fundamental principle has significant implications for our understanding of biology, with potential applications in medicine, sports science, and various other fields. Continued research into cellular energy dynamics promises further advancements that could benefit society as a whole.
.jpg)
.jpg)

.PNG)