Does The Electron Transport Chain Require Oxygen

The electron transport chain (ETC), a critical component of cellular respiration, is often presented as directly dependent on oxygen. But is this universally true? Examining the nuances of the ETC reveals a more complex relationship, one with significant implications for understanding life in diverse environments and developing new biotechnologies.
This article explores the role of oxygen in the electron transport chain, examining instances where it is essential and alternative pathways that function without it. Understanding these variations is crucial for appreciating the adaptability of living organisms and their ability to thrive in oxygen-poor conditions. We will explore the biological necessity of oxygen.
The Conventional View: Oxygen as the Terminal Electron Acceptor
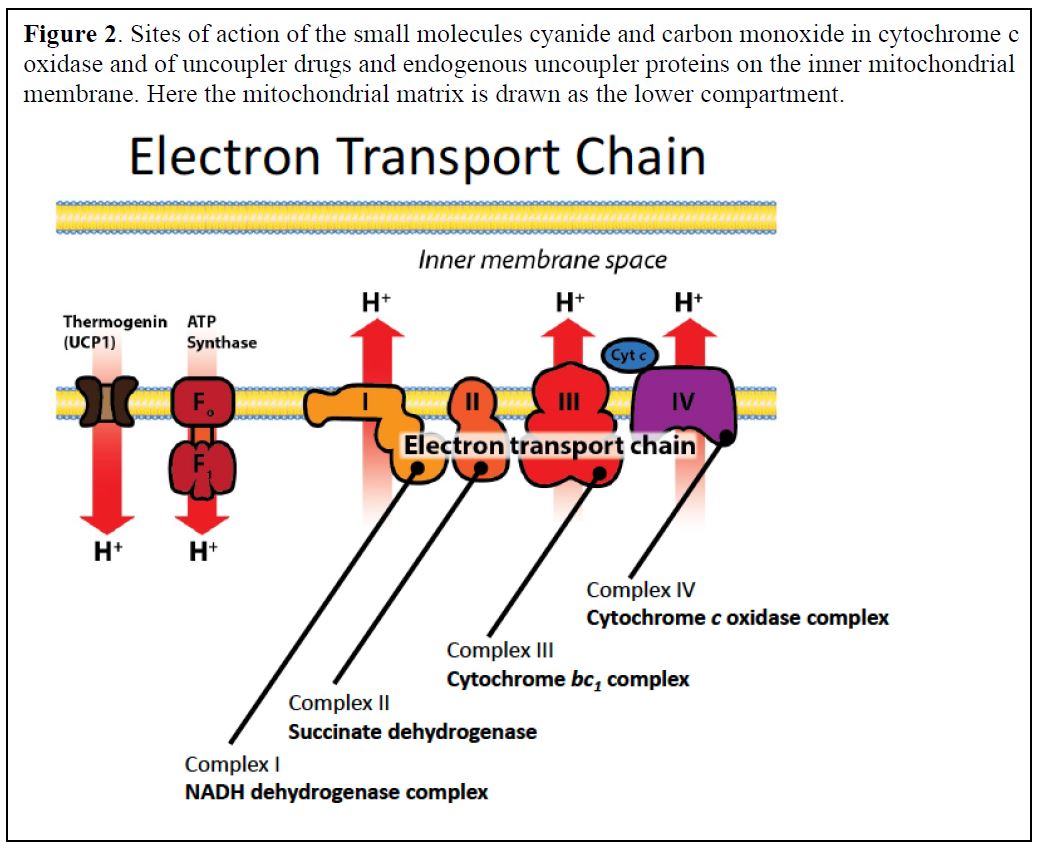
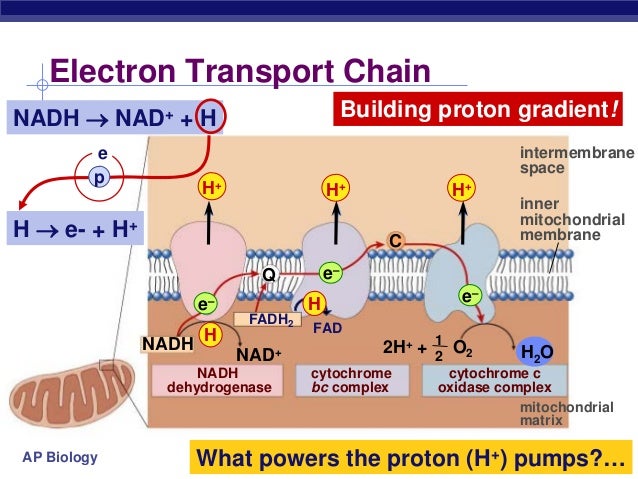
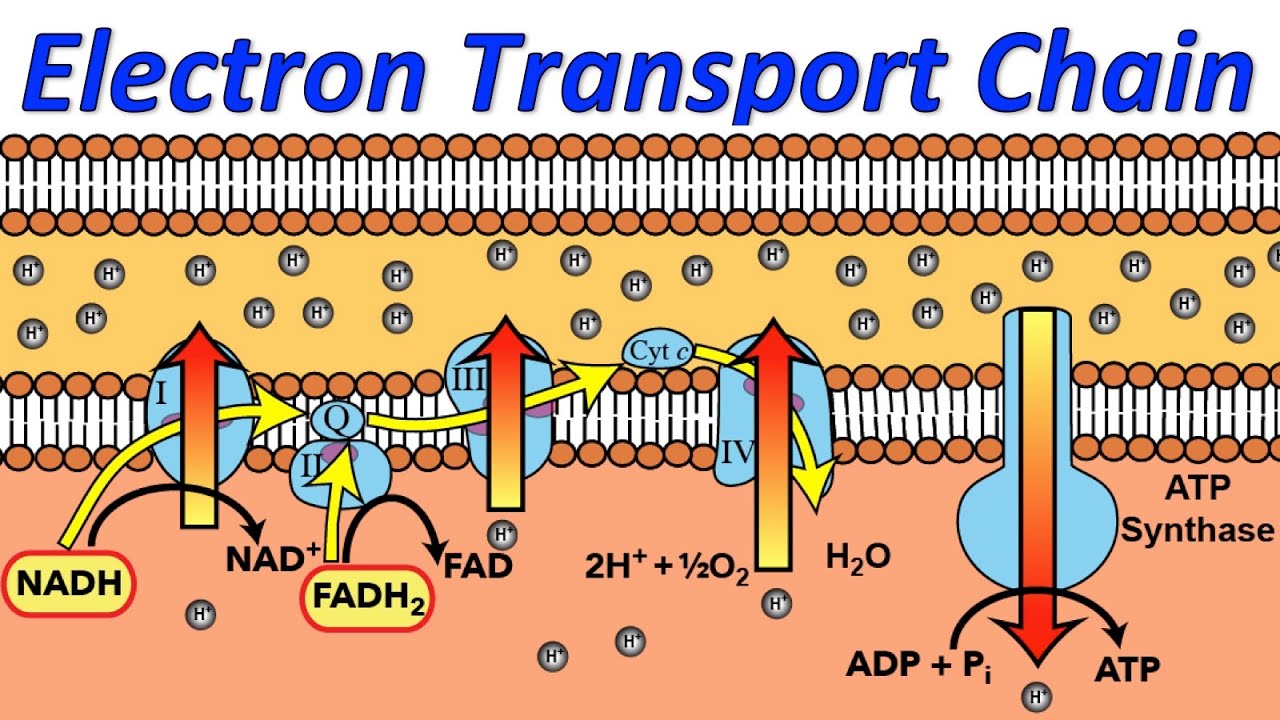
The electron transport chain, located within the mitochondria of eukaryotic cells and the cell membrane of prokaryotes, is the final stage of aerobic respiration. Its primary function is to generate a proton gradient across the inner mitochondrial membrane (or cell membrane) which drives ATP synthesis via oxidative phosphorylation.
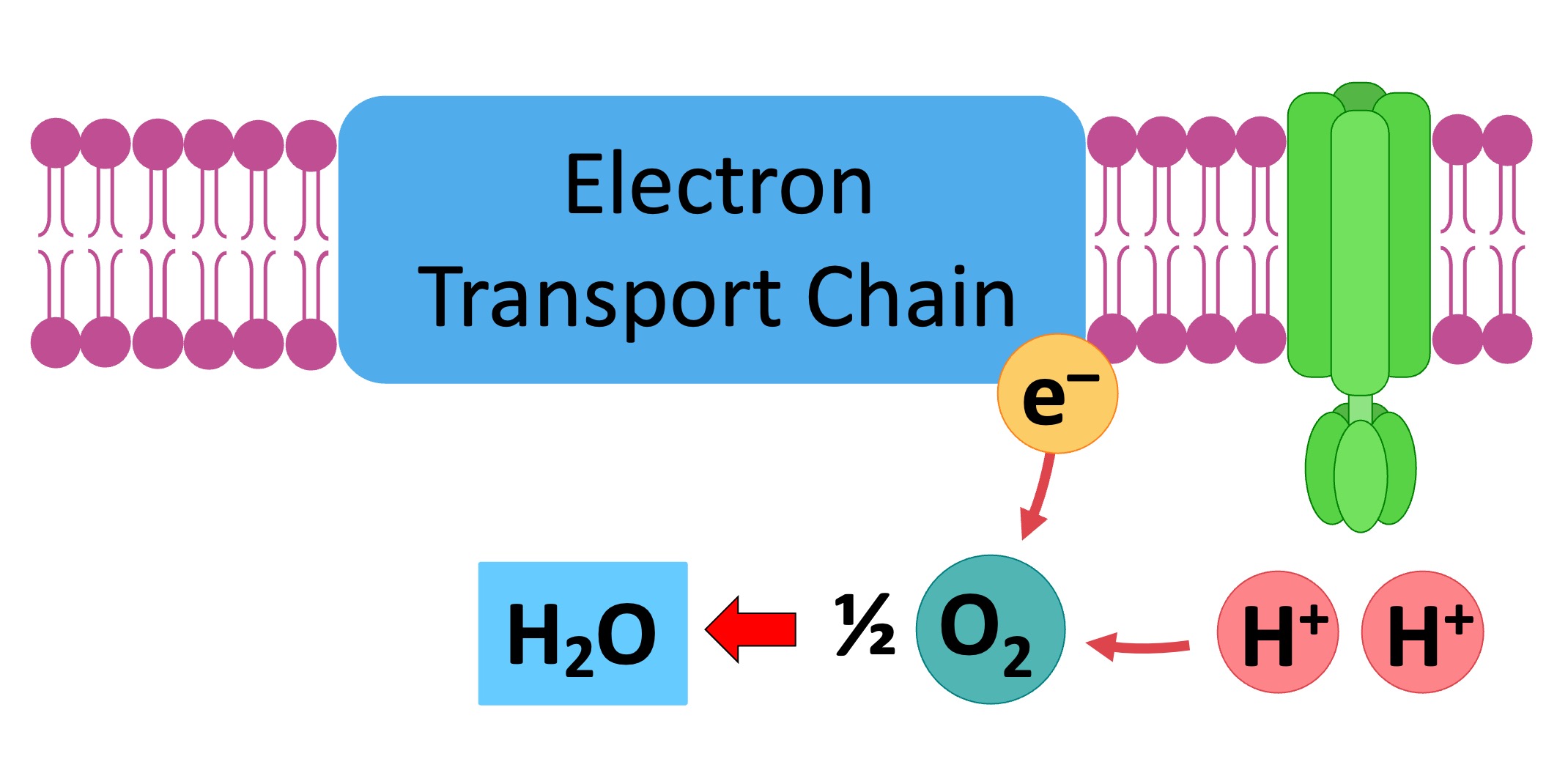
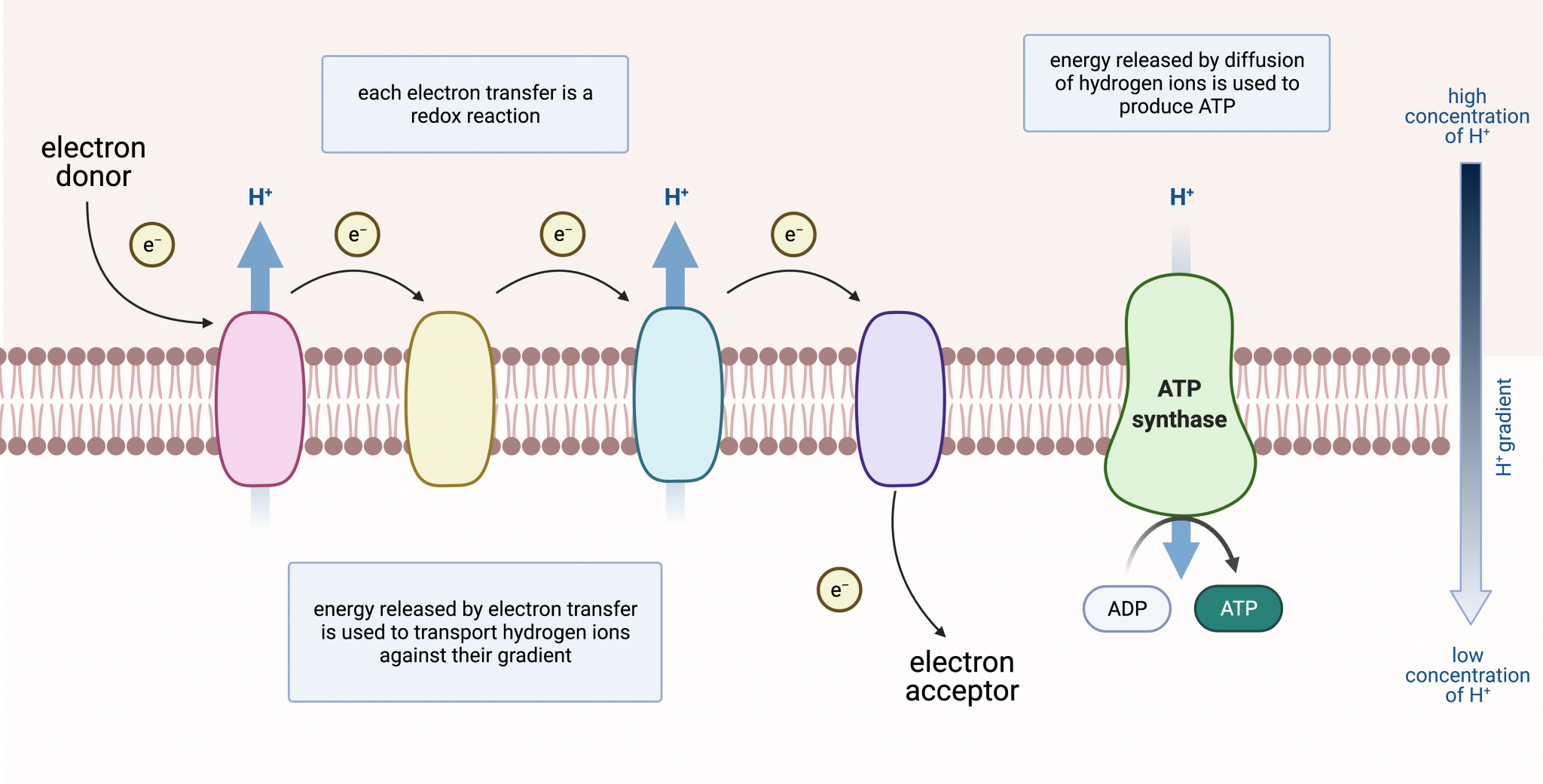
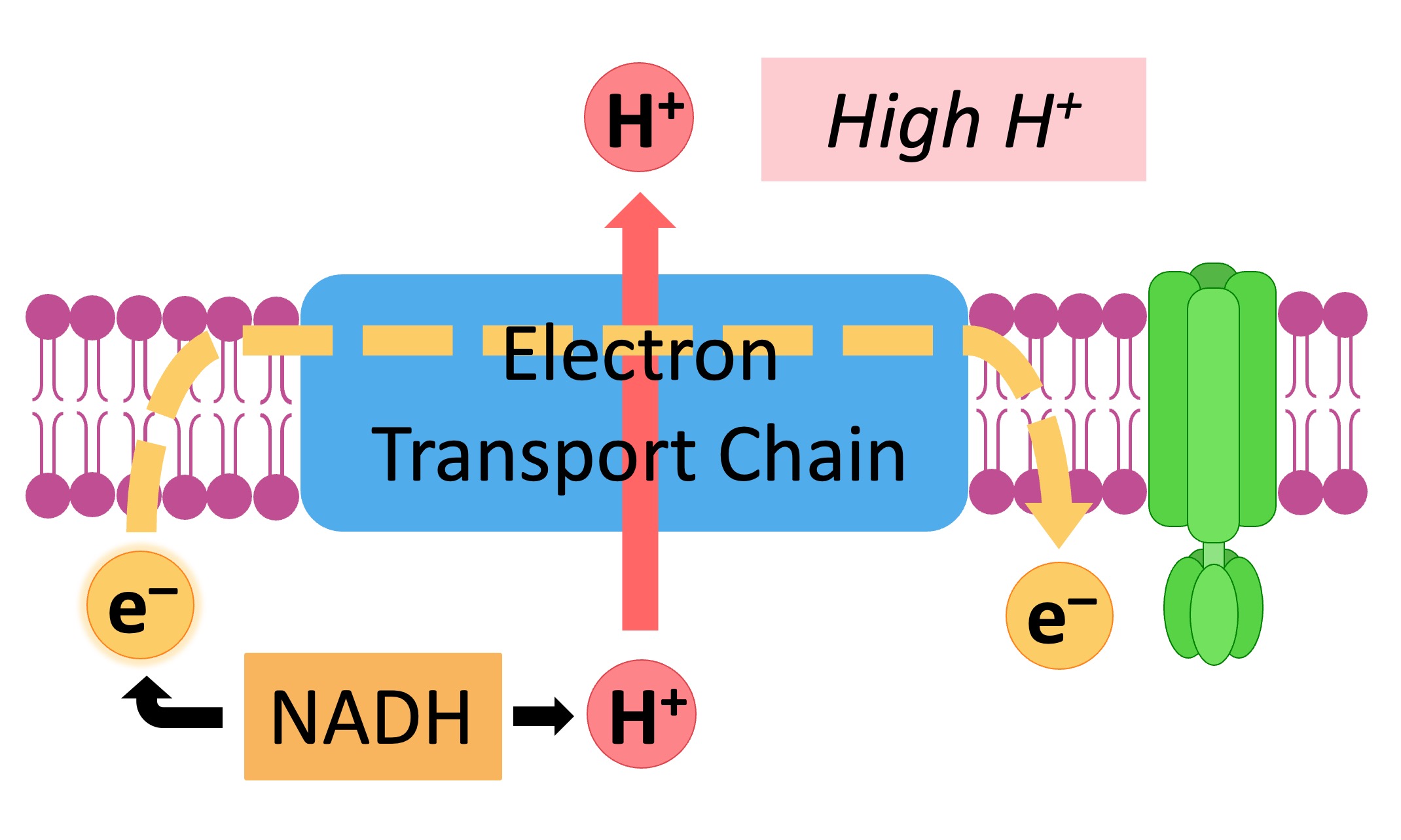
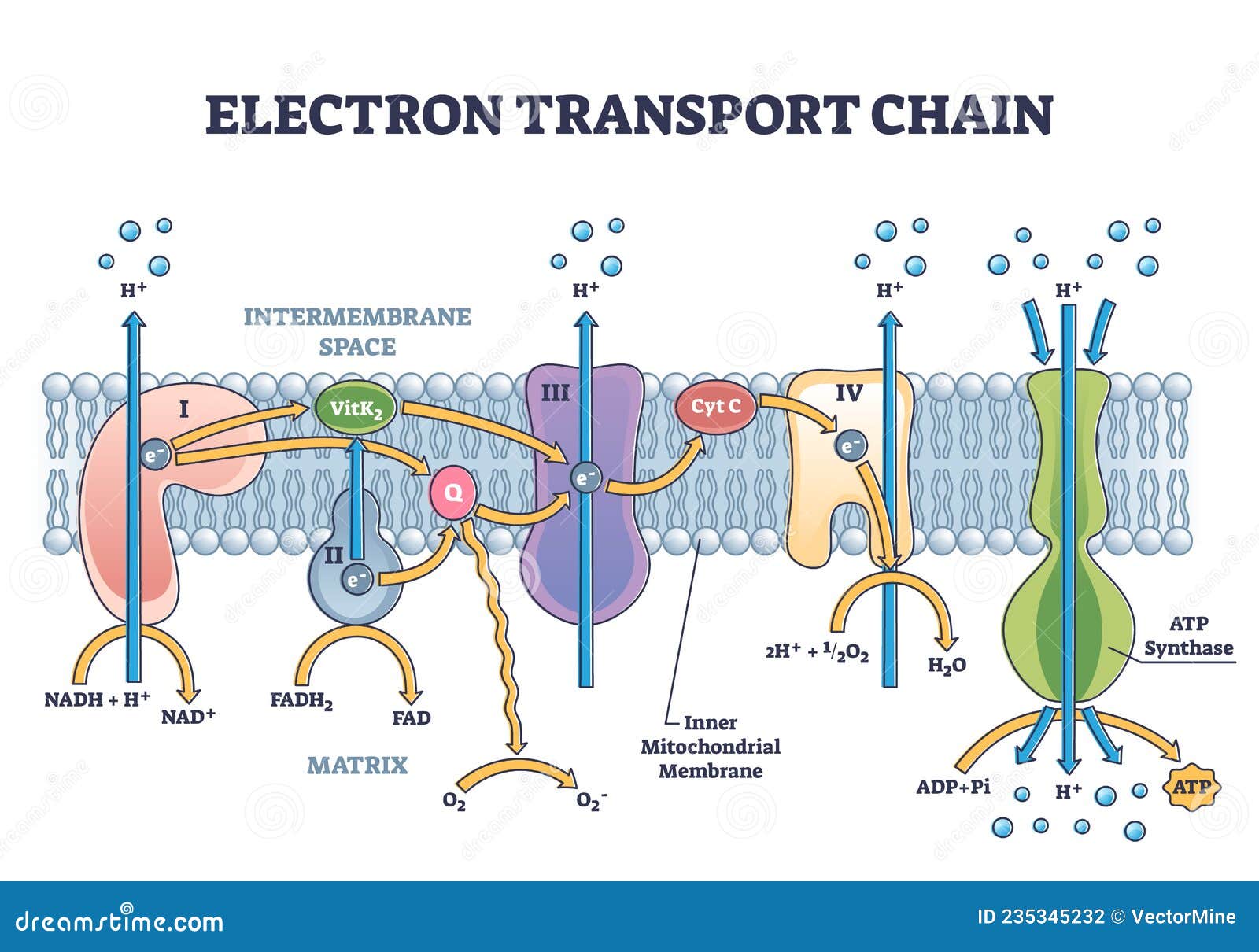
In the classical model, oxygen acts as the terminal electron acceptor in the ETC. Electrons are passed down a series of protein complexes (Complex I, II, III, and IV) releasing energy at each step.
This energy is used to pump protons (H+) from the mitochondrial matrix to the intermembrane space, creating an electrochemical gradient. Complex IV, also known as cytochrome c oxidase, catalyzes the final transfer of electrons to oxygen, reducing it to water (H2O). This process is vital for maintaining the flow of electrons through the chain and preventing its backup.
Anaerobic Respiration: Life Beyond Oxygen
While oxygen is the most efficient terminal electron acceptor, it is not the only option. Many microorganisms, particularly bacteria and archaea, employ anaerobic respiration, utilizing alternative electron acceptors such as nitrate (NO3-), sulfate (SO42-), iron (Fe3+), or carbon dioxide (CO2).
These alternative electron acceptors have a lower reduction potential than oxygen, meaning that less energy is released during electron transfer. As a result, anaerobic respiration generally yields less ATP than aerobic respiration.
However, in environments where oxygen is scarce or absent, such as deep-sea sediments, anaerobic respiration is essential for survival. Denitrifying bacteria, for instance, use nitrate as a terminal electron acceptor, converting it to nitrogen gas (N2).
Examples of Anaerobic ETC Pathways
Several well-characterized anaerobic respiratory pathways exist. Sulfate-reducing bacteria utilize sulfate as a terminal electron acceptor, producing hydrogen sulfide (H2S), a toxic gas responsible for the rotten egg smell often associated with anaerobic environments.
Methanogens, a group of archaea, use carbon dioxide as a terminal electron acceptor, reducing it to methane (CH4). This process is crucial in the carbon cycle and contributes significantly to global methane emissions.
Even some bacteria can use iron (Fe3+) as the terminal electron acceptor, reducing it to Fe2+. This can be important for iron cycling in soils and sediments.
The Implications of Oxygen-Independent ETCs
The existence of anaerobic electron transport chains has profound implications for understanding the diversity of life on Earth and its potential in other environments. It expands our understanding of the limits of life and the range of metabolic strategies that organisms can employ.
Furthermore, understanding these pathways has biotechnological potential. For example, manipulating anaerobic respiration could be used to bioremediate contaminated sites by removing pollutants or producing valuable compounds.
Moreover, the study of anaerobic ETCs is crucial in astrobiology, informing the search for life on other planets where oxygen may be absent. Understanding how organisms can thrive in oxygen-deprived environments on Earth gives us clues about the potential for life to exist in similar environments elsewhere in the universe.
Conclusion: A More Nuanced Perspective
The electron transport chain is not exclusively dependent on oxygen. While oxygen is the most efficient terminal electron acceptor and is crucial for aerobic organisms, many microorganisms can thrive in the absence of oxygen by utilizing alternative electron acceptors.
The study of these anaerobic ETCs provides valuable insights into the adaptability of life and opens up new avenues for biotechnological applications and astrobiological exploration. This understanding challenges the simple narrative that oxygen is an absolute requirement for the ETC and highlights the remarkable metabolic diversity that exists in the biological world.
Therefore, while often presented as an oxygen-dependent process, the electron transport chain exhibits remarkable flexibility, adapting to diverse environmental conditions and utilizing a range of terminal electron acceptors to sustain life even in the absence of oxygen.


.jpg)
.jpg)
![Does The Electron Transport Chain Require Oxygen [Figure, Electron Transport Chain graphic. Shows...] - StatPearls](https://www.ncbi.nlm.nih.gov/books/NBK526105/bin/Electron_Transport_Chain__2.jpg)