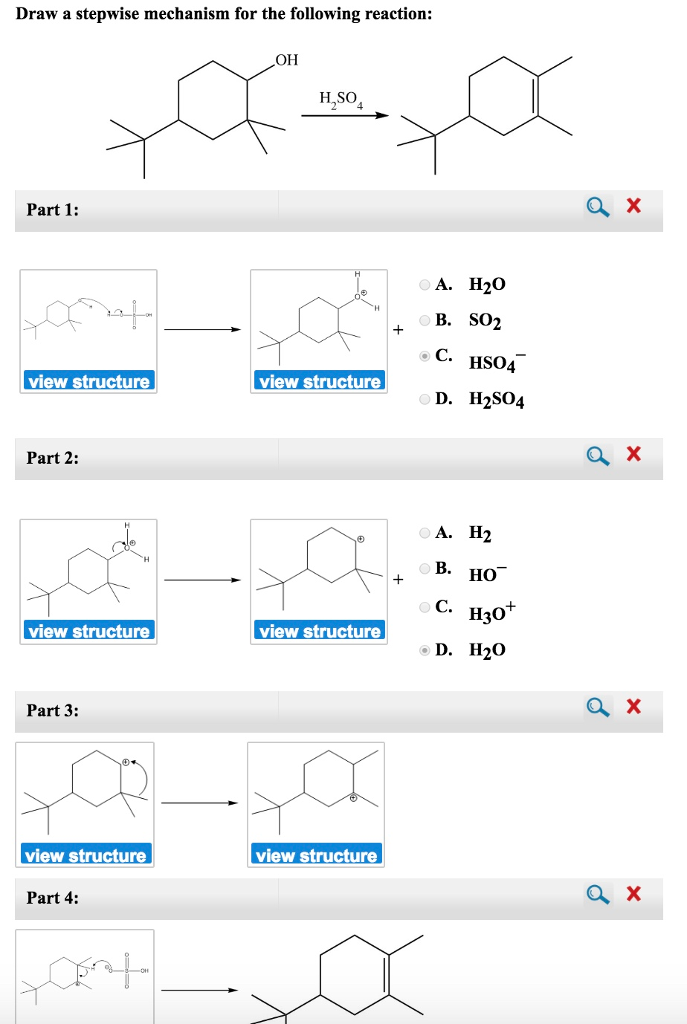
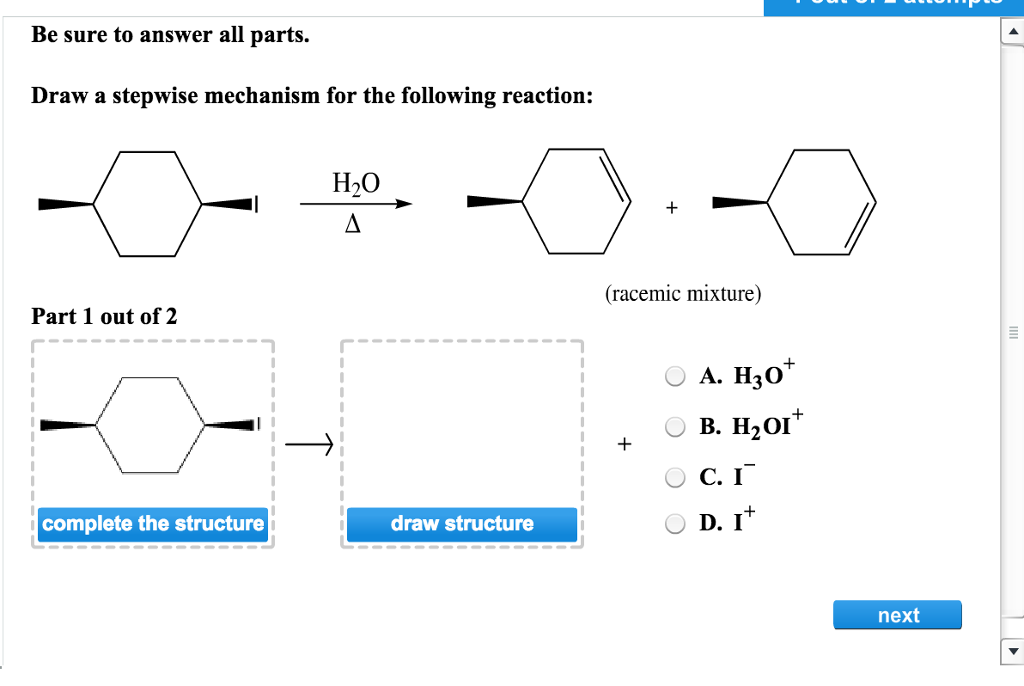
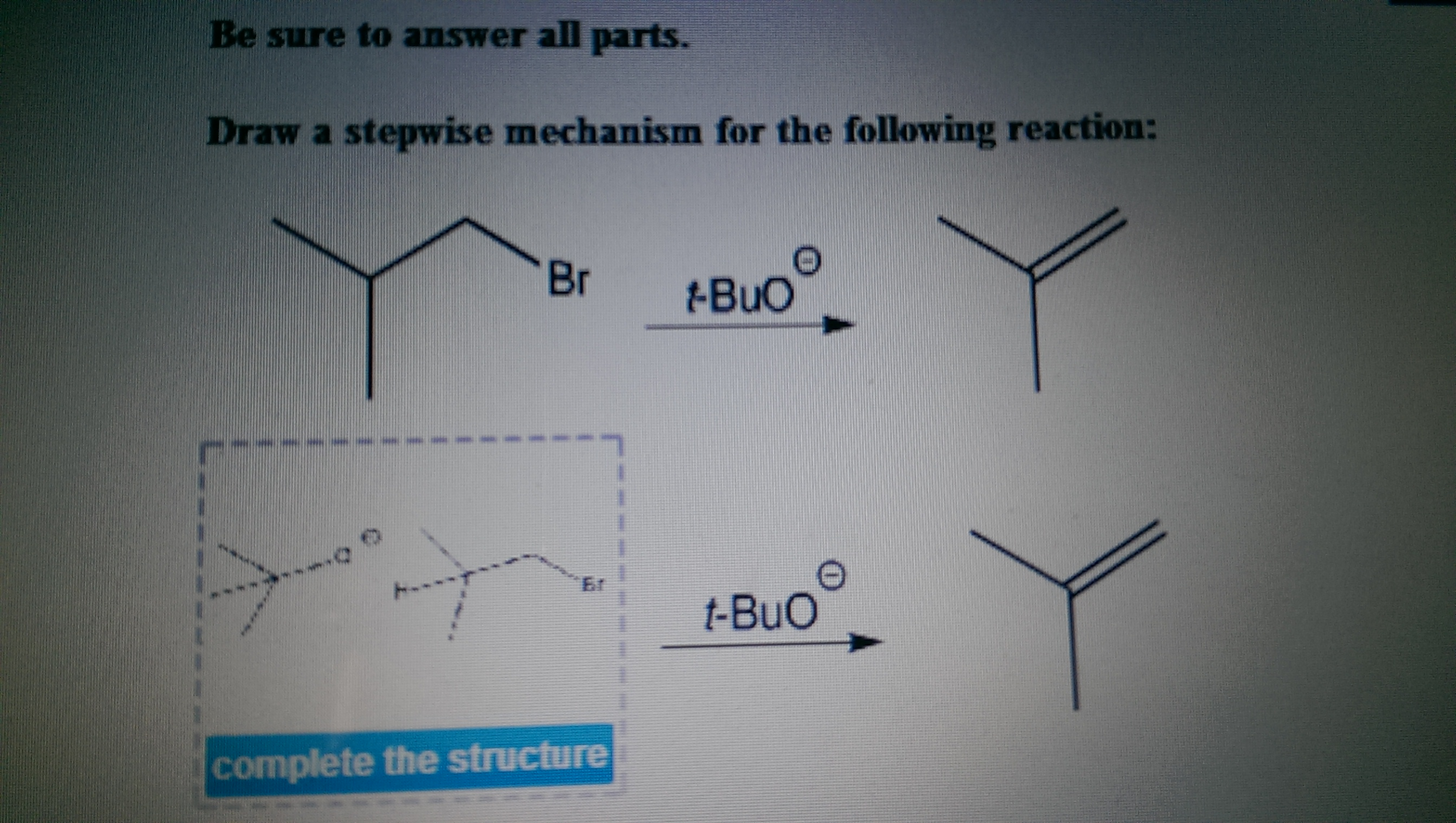
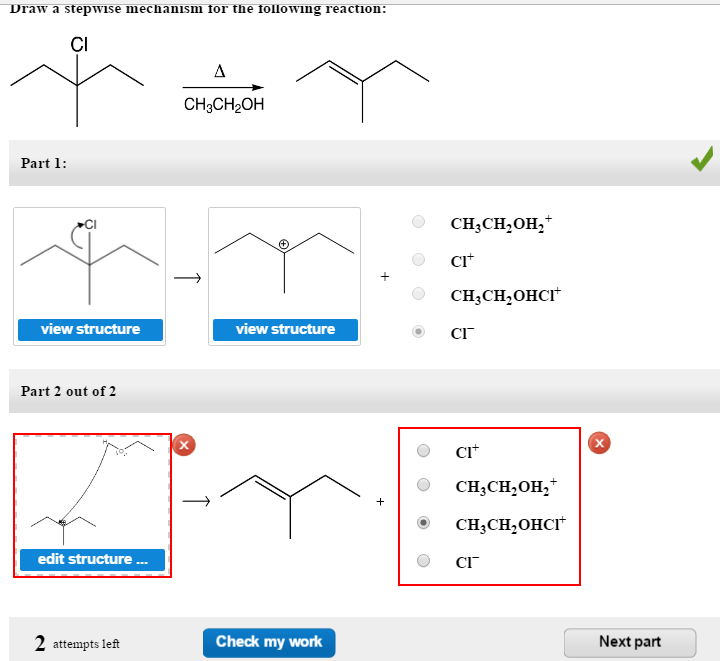
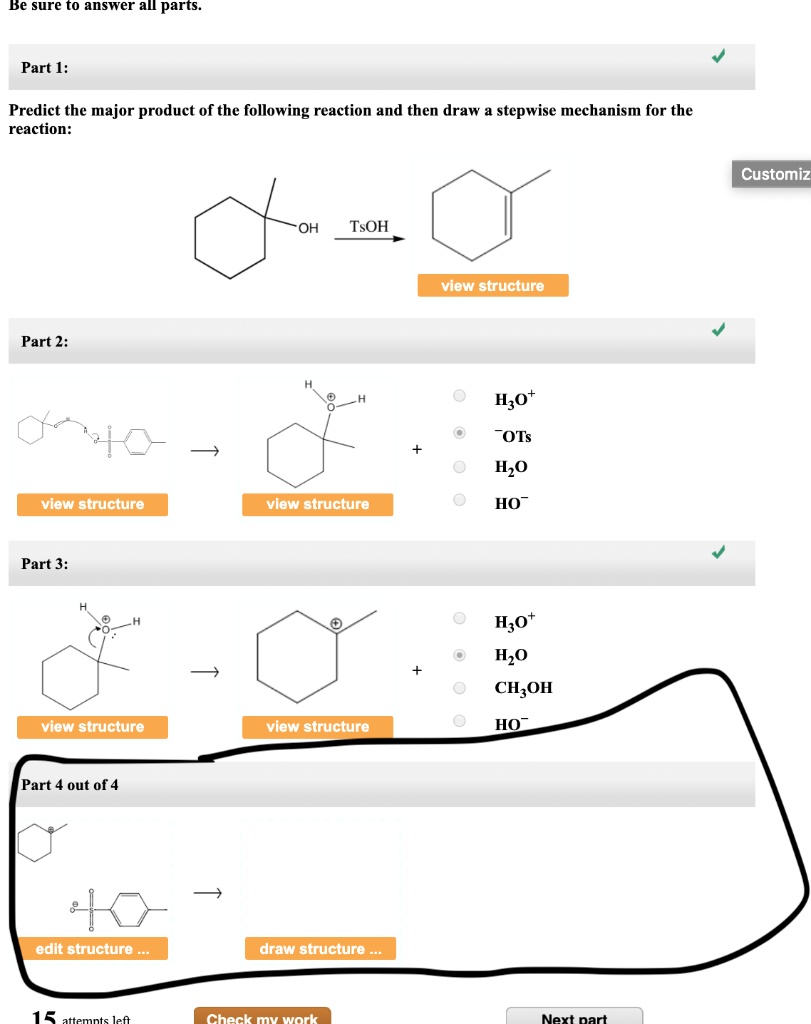
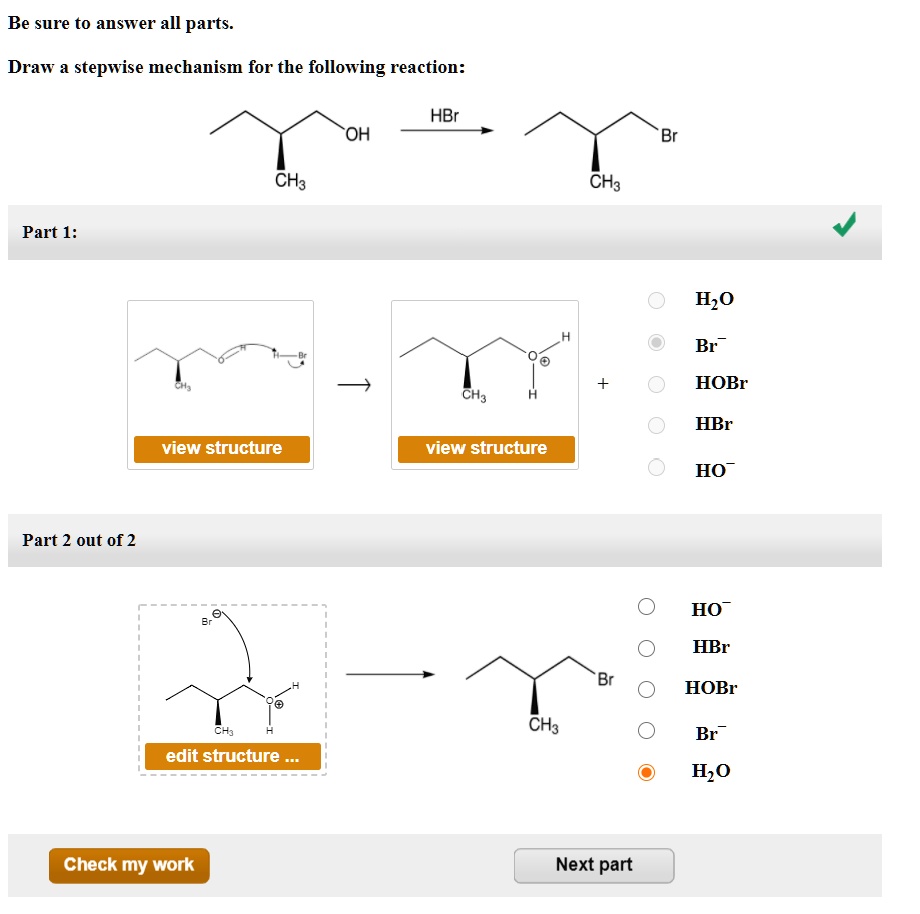
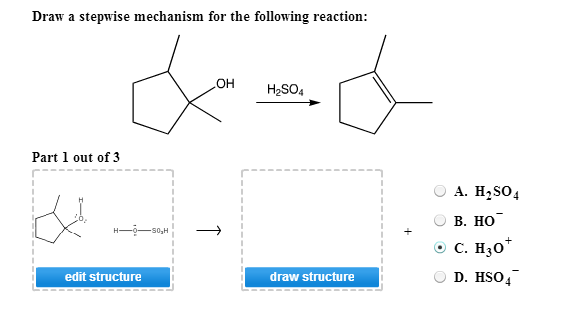
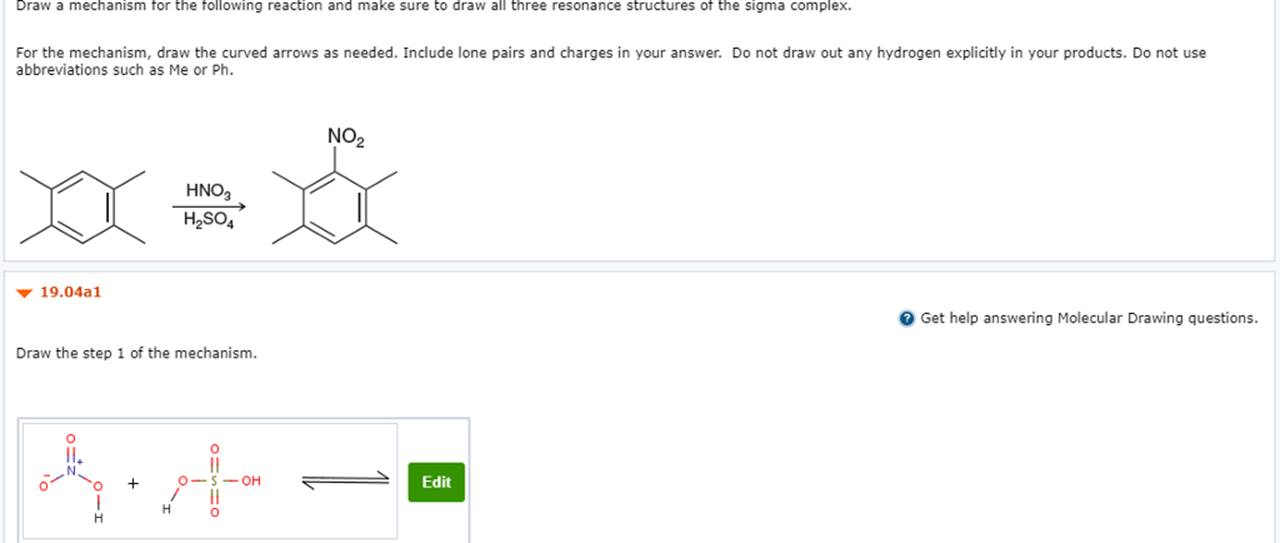
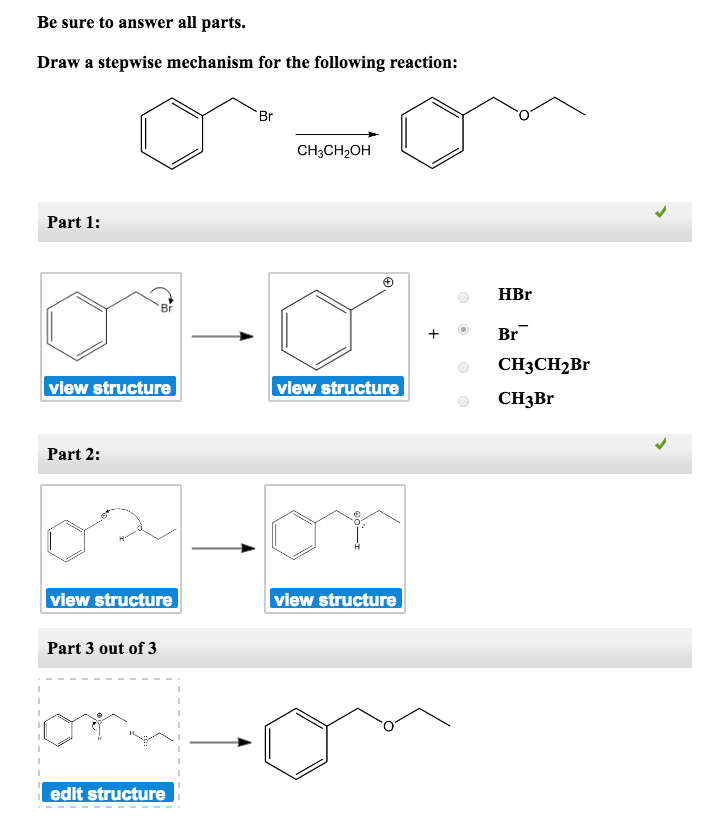
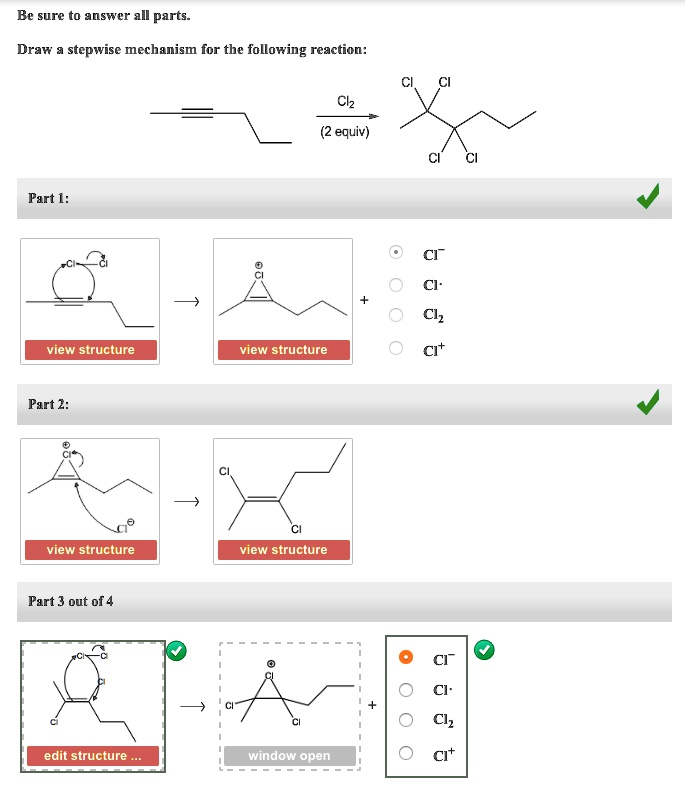
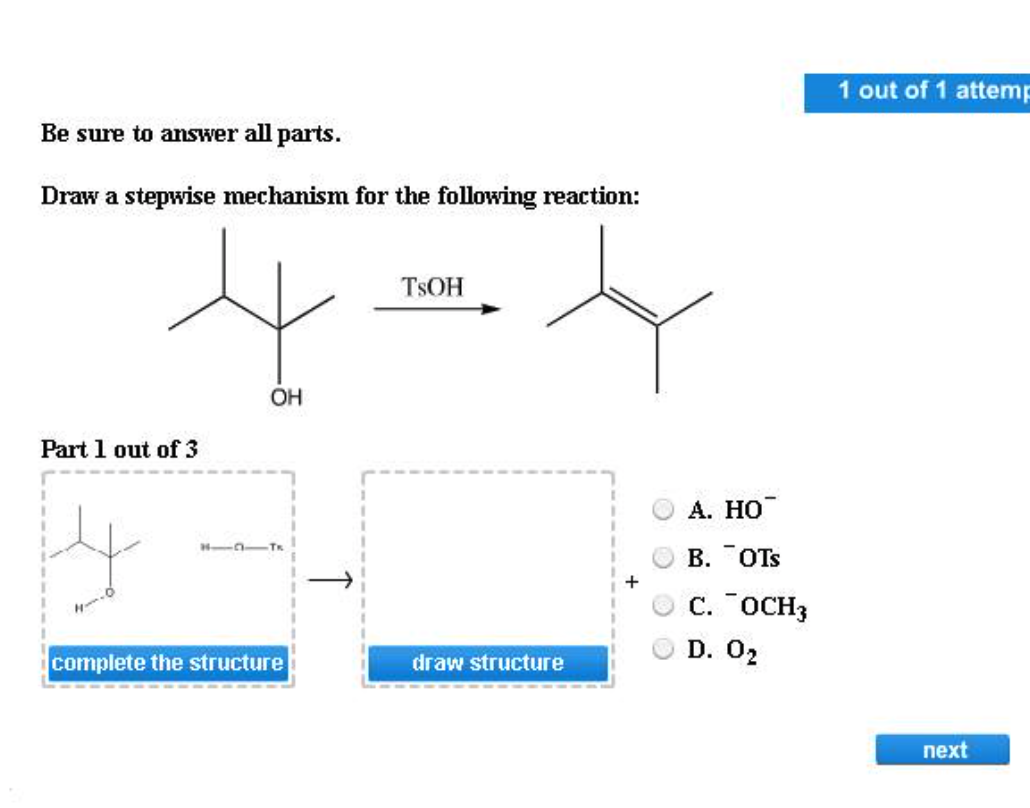
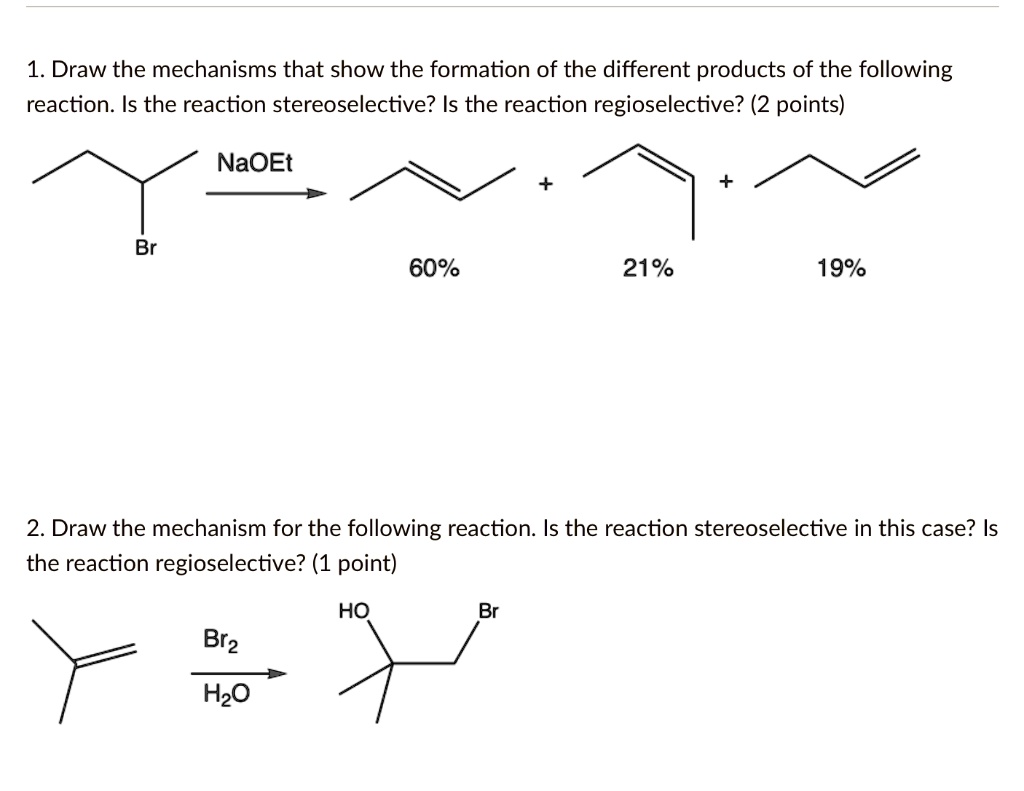
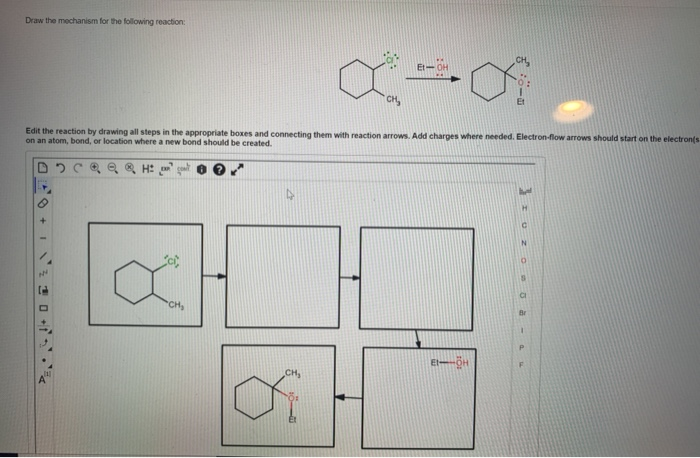
Draw The Mechanism For The Following Reaction
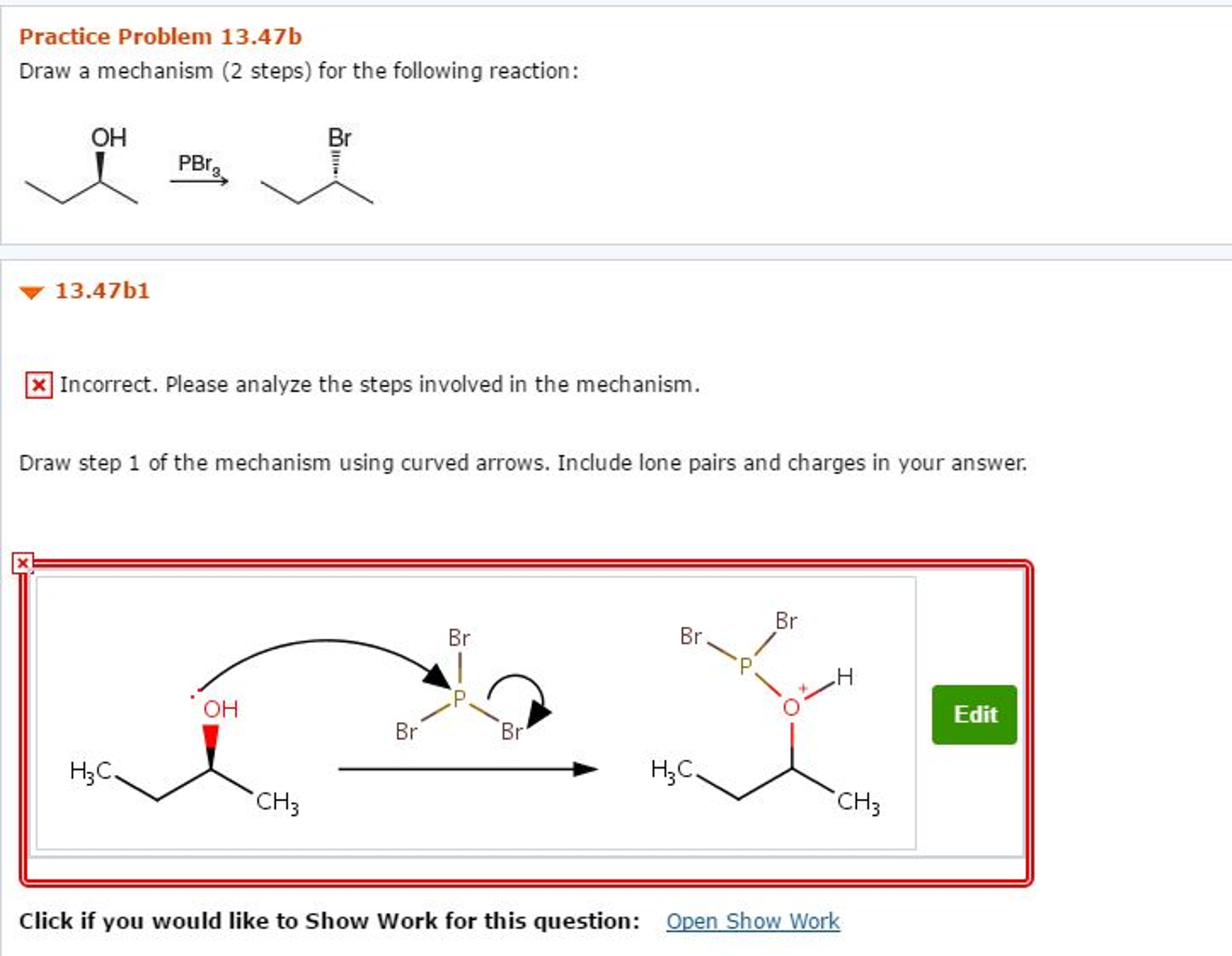
The academic world, particularly the realm of organic chemistry, is abuzz. A specific reaction mechanism, presented in a recent examination, has sparked widespread debate and scrutiny. Students and professors alike are dissecting its nuances, attempting to fully elucidate the step-by-step process.
The core of the controversy revolves around the proposed mechanism for a complex organic transformation. This reaction, while seemingly straightforward at first glance, presents several challenges in terms of understanding its intermediate steps, transition states, and overall energetic profile.
The Reaction at the Heart of the Debate
While the specific reaction cannot be reproduced here, it involves the conversion of one organic molecule into another, facilitated by specific reagents and conditions. The suggested mechanism outlines the sequence of bond-breaking and bond-forming events that lead to the product.
The primary point of contention lies in the proposed order of these events. Several alternative pathways are being considered by chemists, each with its own set of supporting arguments and potential drawbacks.
The crux of the matter is determining the most energetically favorable route for the reaction to proceed. This involves evaluating the stability of various intermediates and the activation energies associated with each step.
Expert Opinions and Diverging Perspectives
We reached out to several leading figures in the field of organic chemistry for their insights on this perplexing reaction. Professor Anya Sharma, a renowned expert in reaction mechanisms, expressed reservations about the initially proposed pathway.
“While the suggested mechanism is plausible on paper, certain steps seem unlikely based on our current understanding of chemical kinetics and thermodynamics,” Professor Sharma stated. She highlights the potential for alternative intermediates and the influence of solvent effects, which could significantly alter the reaction pathway.
Conversely, Dr. Ben Carter, a computational chemist specializing in reaction modeling, presented a different viewpoint. "Our simulations suggest that the original mechanism is indeed feasible, although it might not be the only possible route," Dr. Carter explained.
His research indicates that the transition states involved in the proposed mechanism are within an acceptable energy range. However, he acknowledges that further investigation is needed to fully account for all potential contributing factors.
The Importance of Experimental Validation
Ultimately, the validity of any proposed reaction mechanism hinges on experimental evidence. This includes conducting kinetic studies to determine the rate-determining step and identifying any detectable intermediates through spectroscopic techniques.
Furthermore, isotopic labeling experiments can provide crucial information about the fate of specific atoms during the reaction. These experiments help to confirm or refute the proposed bond-breaking and bond-forming events.
"Theoretical calculations can provide valuable insights, but they must be validated by empirical data," emphasized Professor Sharma. The interplay between theory and experiment is essential for a comprehensive understanding of any chemical reaction.
Potential Pitfalls and Alternative Mechanisms
One of the main criticisms of the initial mechanism involves a potentially unstable intermediate. This intermediate, if formed, could lead to undesired side reactions or a drastically slower reaction rate.
As a result, several alternative mechanisms have been proposed, each attempting to circumvent this potential bottleneck. These alternative pathways often involve different reactive species or a different sequence of events.
Another point of contention is the role of the solvent. Depending on the polarity and proticity of the solvent, the reaction pathway could be significantly altered. This is especially true for reactions involving charged intermediates or transition states.
Implications for Future Research and Education
The ongoing debate surrounding this reaction highlights the complexity and ever-evolving nature of organic chemistry. It underscores the importance of critical thinking, rigorous experimentation, and a willingness to challenge established assumptions.
This case study provides a valuable learning opportunity for students. It illustrates the process of developing, evaluating, and refining reaction mechanisms, and showcases the collaborative nature of scientific inquiry.
By critically analyzing the proposed mechanism and exploring alternative pathways, students can develop a deeper understanding of the fundamental principles that govern chemical reactions.
Moving Forward: Collaboration and Continued Investigation
To resolve the current ambiguities surrounding this reaction, a collaborative effort involving both experimentalists and computational chemists is crucial. Further experimental studies are needed to identify any detectable intermediates and to determine the kinetic parameters of the reaction.
Simultaneously, more sophisticated computational models can be employed to explore the potential energy surface of the reaction and to identify the most likely reaction pathway. The synthesis of specifically designed molecules to probe individual mechanistic steps would add more value.
Only through a combined approach can we hope to fully elucidate the intricacies of this reaction and to arrive at a definitive understanding of its mechanism. The outcome of this debate will not only refine our understanding of this specific reaction but will also contribute to a broader understanding of organic reactivity.