Five Chemical Properties Of Carboxylic Acid
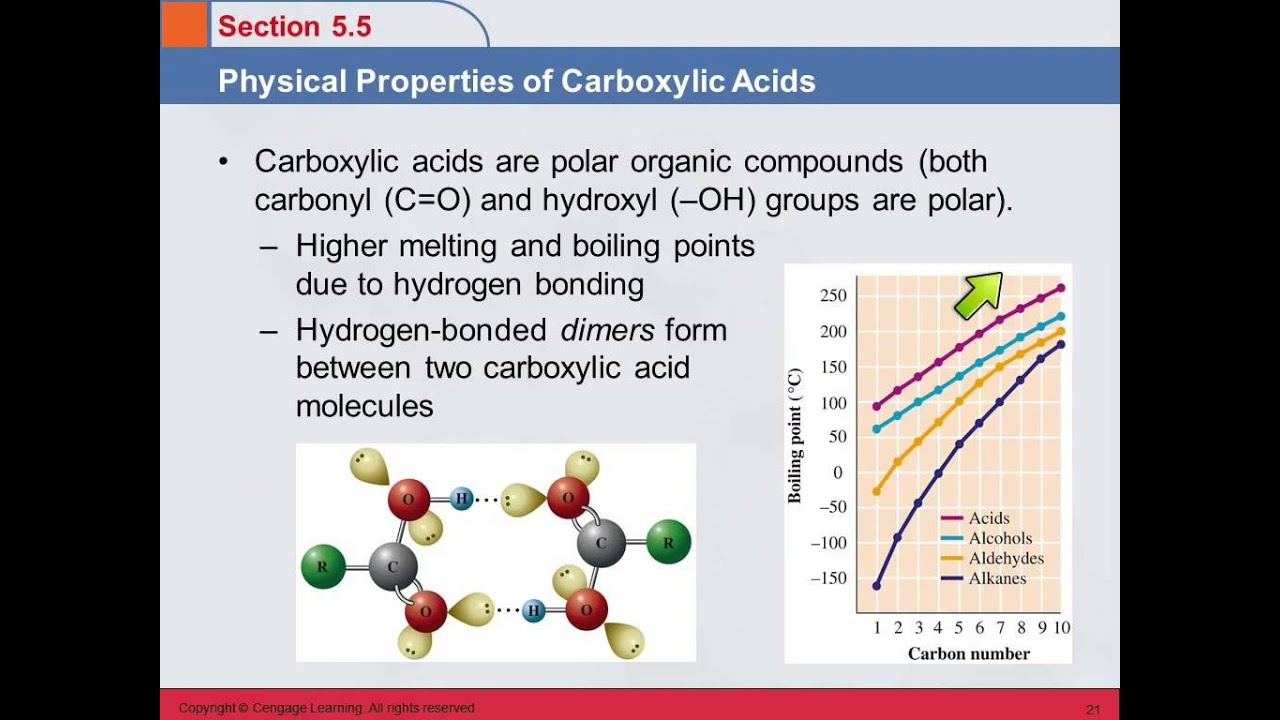
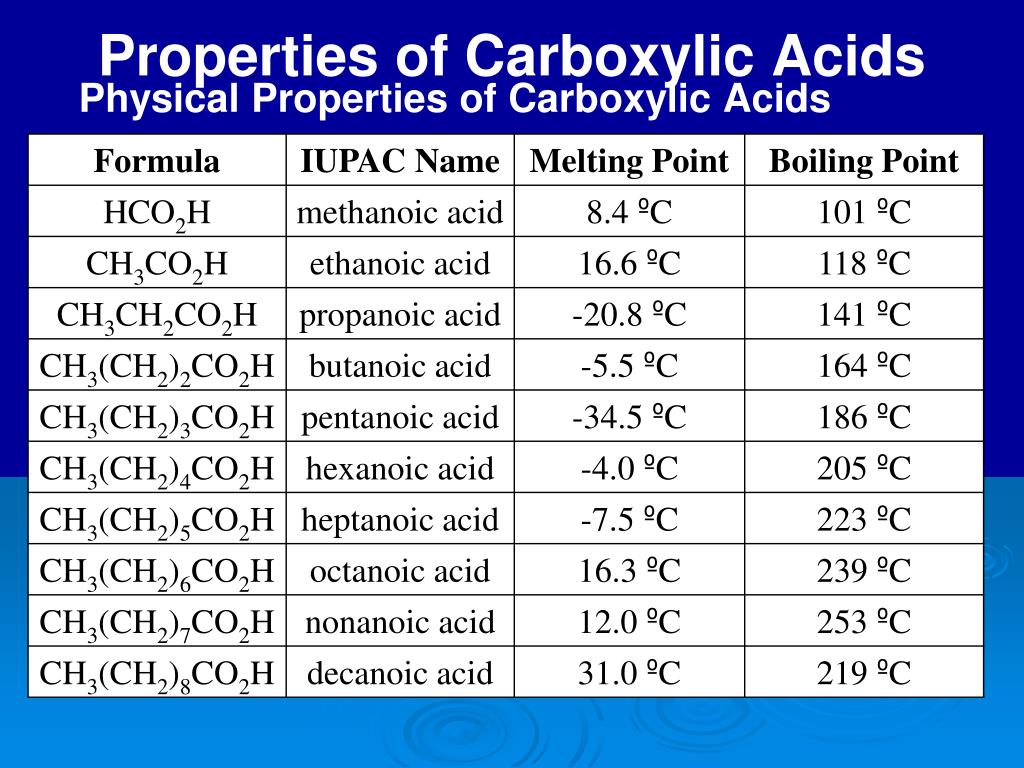
The unseen world of molecules dictates the behavior of our world, and within this realm, carboxylic acids hold a position of particular importance. These organic compounds, found everywhere from the simplest vinegar in our kitchens to the complex proteins within our bodies, owe their diverse functions to a set of key chemical properties. Understanding these properties is critical for advancing fields ranging from pharmaceuticals to materials science.
This article delves into five fundamental chemical properties of carboxylic acids: acidity, esterification, amide formation, reduction, and decarboxylation. Each of these reactions stems from the unique structure of the carboxyl group (-COOH) and dictates how these molecules interact with their environment. A deeper understanding of these properties enables scientists and engineers to harness the power of carboxylic acids for a variety of applications.
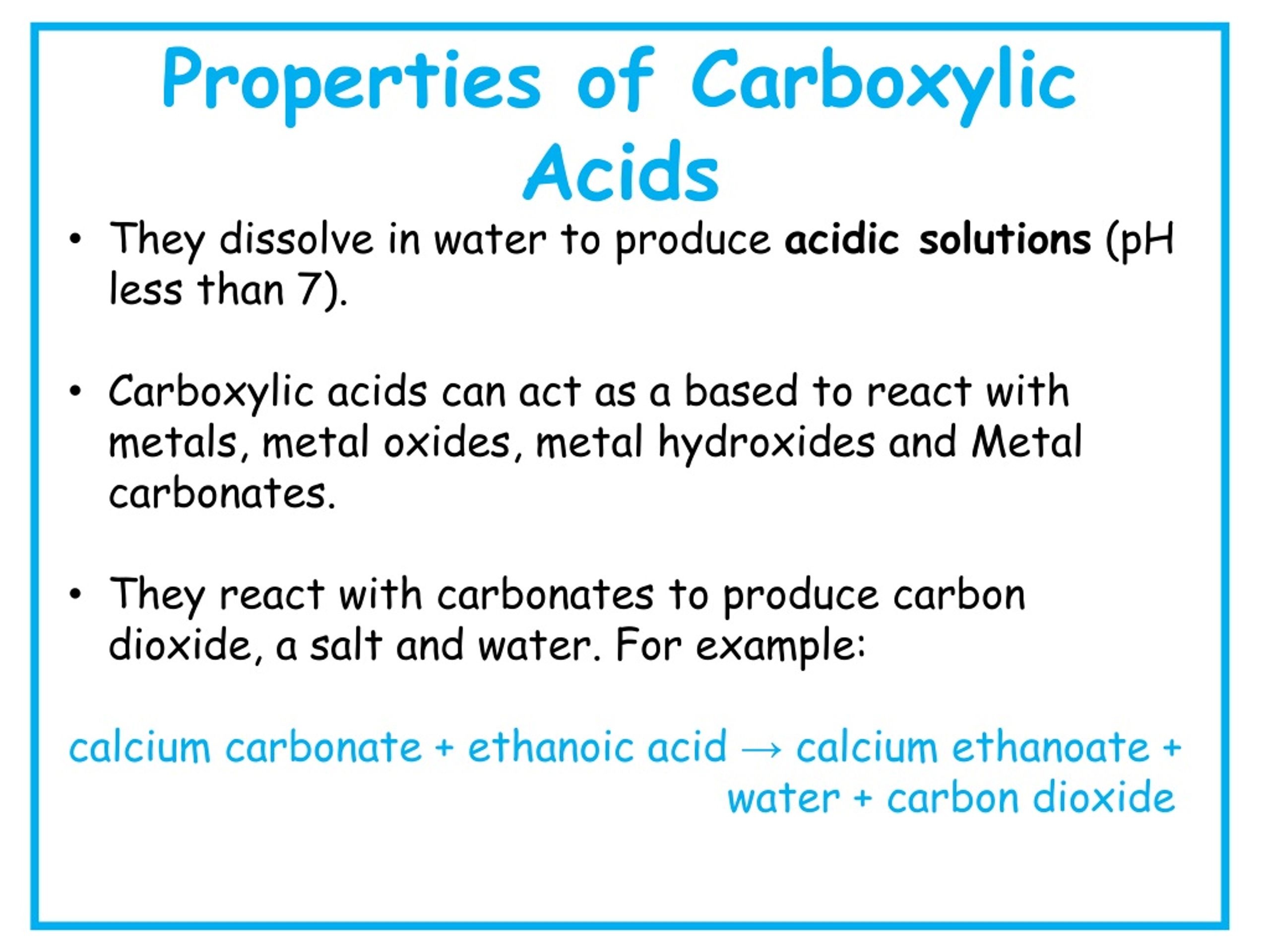
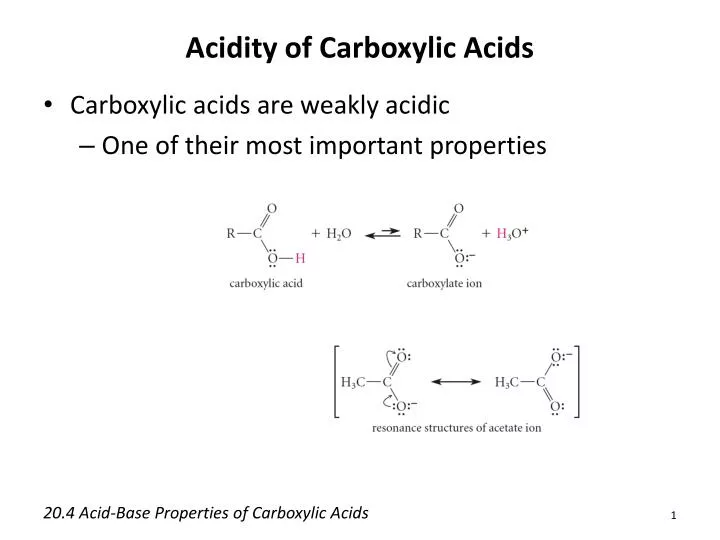
Acidity: A Measure of Proton Donation
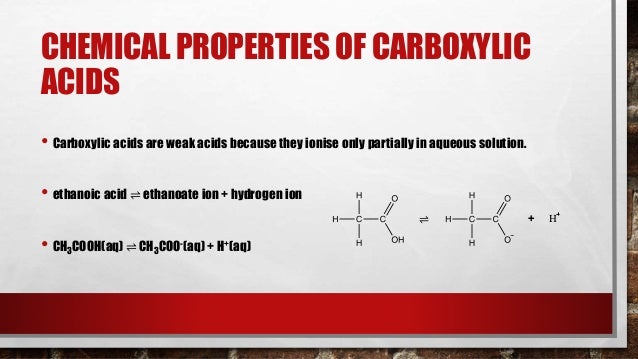
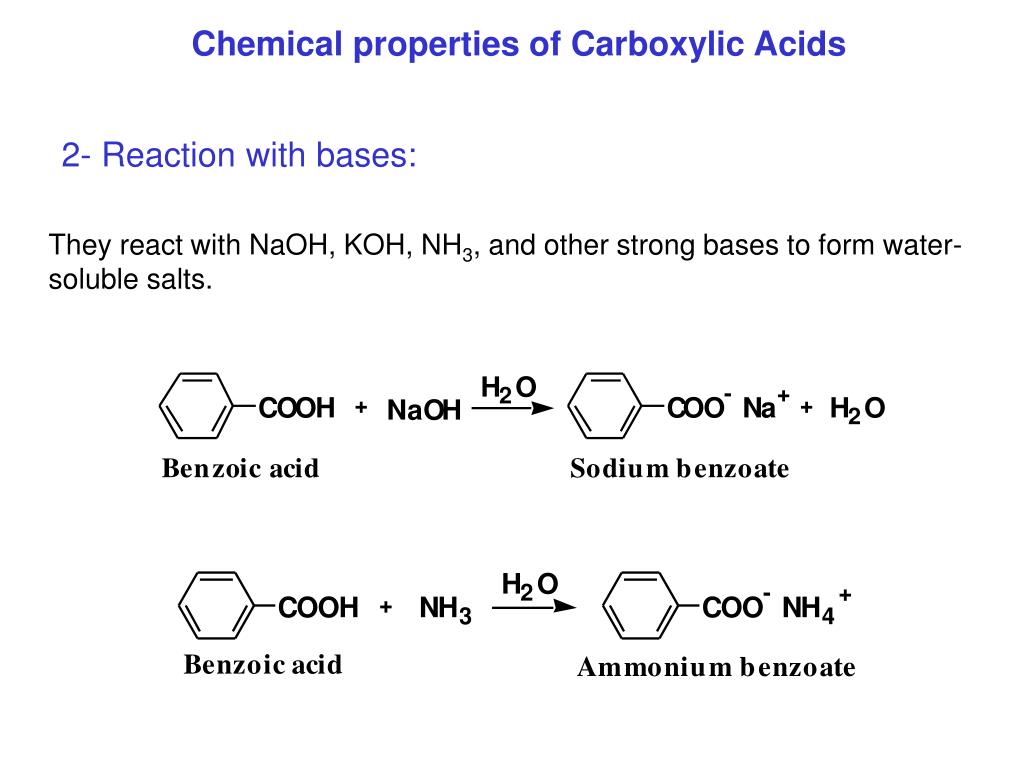
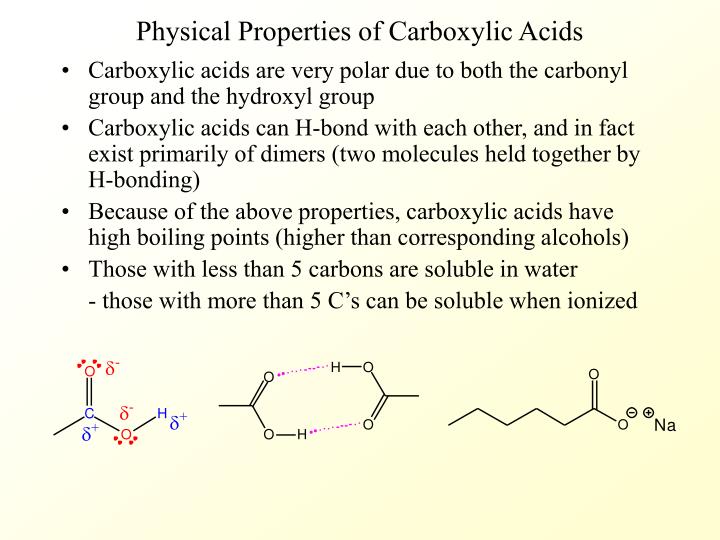
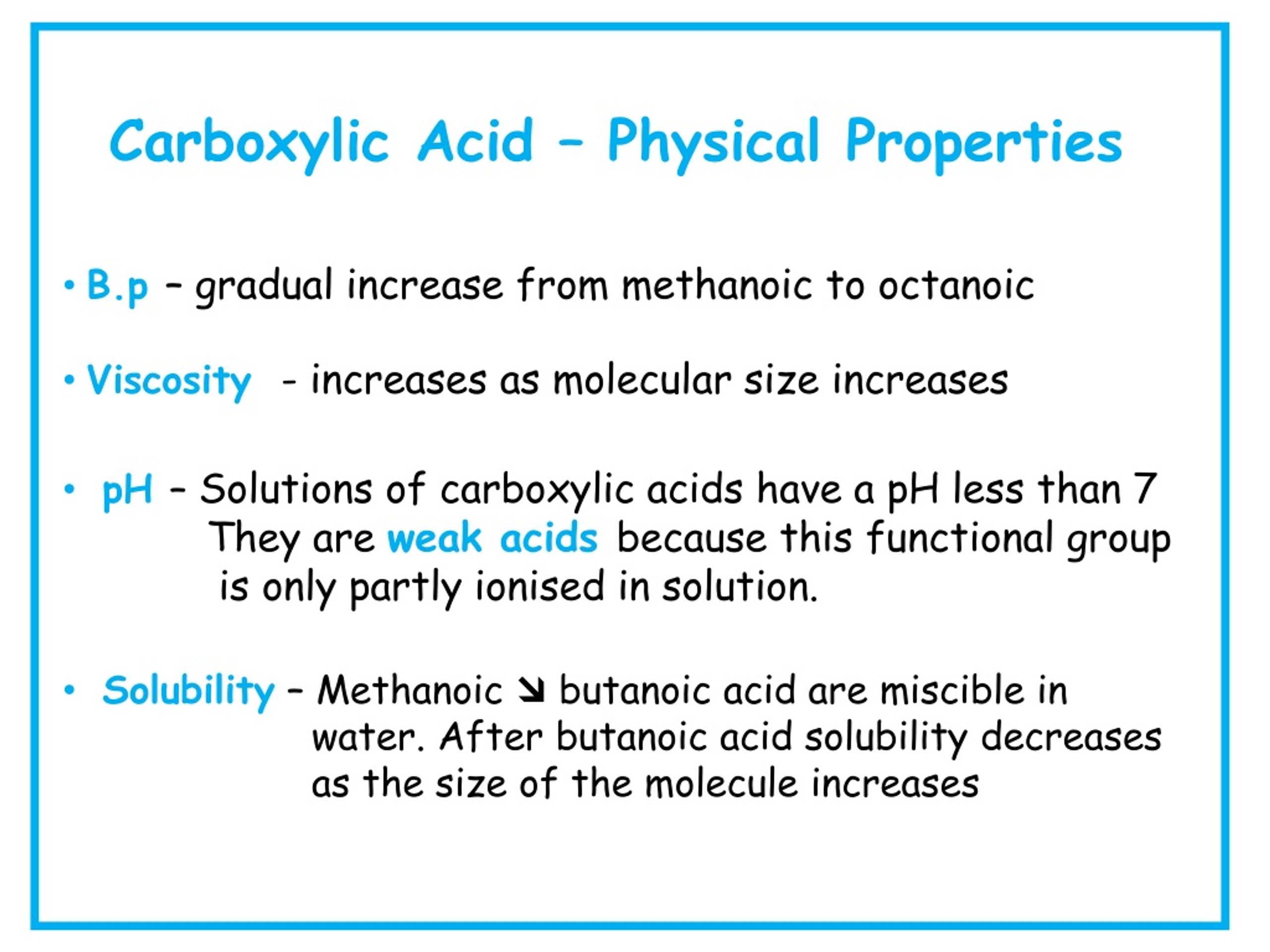
Carboxylic acids are, as the name suggests, acidic in nature. This acidity arises from the ability of the carboxyl group to donate a proton (H+). The resulting carboxylate ion is stabilized by resonance, distributing the negative charge between the two oxygen atoms and making the proton easier to release.
The strength of a carboxylic acid's acidity is quantified by its pKa value. Lower pKa values indicate stronger acids. Factors like electron-withdrawing groups near the carboxyl group can further stabilize the carboxylate ion, increasing the acidity of the carboxylic acid.
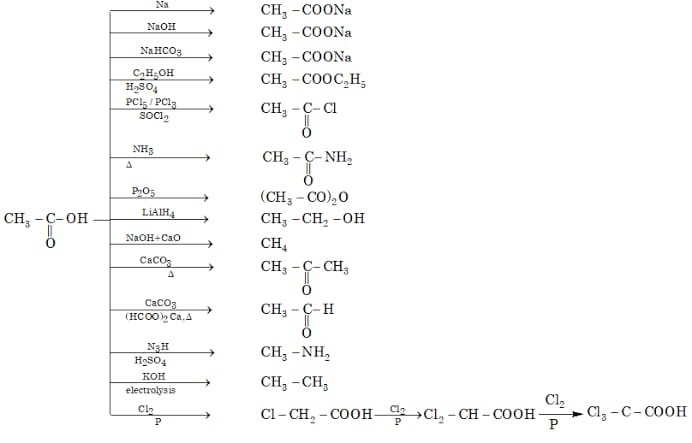
Esterification: Creating Fragrant Compounds
Esterification is the reaction between a carboxylic acid and an alcohol, resulting in the formation of an ester and water. This reaction is typically catalyzed by a strong acid, such as sulfuric acid.
Esters are widely known for their pleasant, often fruity, aromas. They are used extensively in perfumes, flavorings, and as solvents. The specific carboxylic acid and alcohol used determine the resulting ester and its characteristic scent.
Amide Formation: Building Blocks of Proteins
Amides are formed when a carboxylic acid reacts with an amine or ammonia. This reaction involves the replacement of the -OH group of the carboxylic acid with an -NR2 group (where R can be hydrogen or an alkyl group).
This reaction typically requires activation of the carboxylic acid to proceed efficiently. Amide bonds are incredibly stable and are the backbone of proteins, linking amino acids together.
Reduction: From Acid to Alcohol
Carboxylic acids can be reduced to primary alcohols using strong reducing agents. Lithium aluminum hydride (LiAlH4) is a common reagent used for this purpose. This reaction involves the replacement of the two oxygen atoms in the carboxyl group with two hydrogen atoms.
The reduction of a carboxylic acid to an alcohol is a valuable transformation in organic synthesis. It allows for the introduction of alcohol functionality into a molecule.
Decarboxylation: Releasing Carbon Dioxide
Decarboxylation is the process of removing a carboxyl group from a carboxylic acid, releasing carbon dioxide (CO2). This reaction typically requires heat and, in some cases, a catalyst.
Beta-keto acids and malonic acids readily undergo decarboxylation due to the stabilization of the resulting enol or carbanion intermediate. Decarboxylation is important in various biochemical pathways, such as the citric acid cycle.
Understanding these five chemical properties of carboxylic acids is essential for manipulating these versatile molecules in countless applications. From synthesizing new pharmaceuticals to developing advanced materials, the reactivity of the carboxyl group provides a powerful tool for chemists and engineers. Further research into novel catalysts and reaction conditions will continue to expand the possibilities of carboxylic acid chemistry and its impact on various industries.