How Do You Add Hydrogen To Water

Scientists are racing to optimize methods for infusing water with hydrogen. The process, crucial for hydrogen-based energy solutions and potential health applications, hinges on efficient and stable techniques.
Understanding how to effectively introduce and maintain hydrogen (H2) in water is paramount for future innovations. This article breaks down the primary methods being explored, their current limitations, and the ongoing research driving progress.
Electrolysis: Splitting Water Molecules
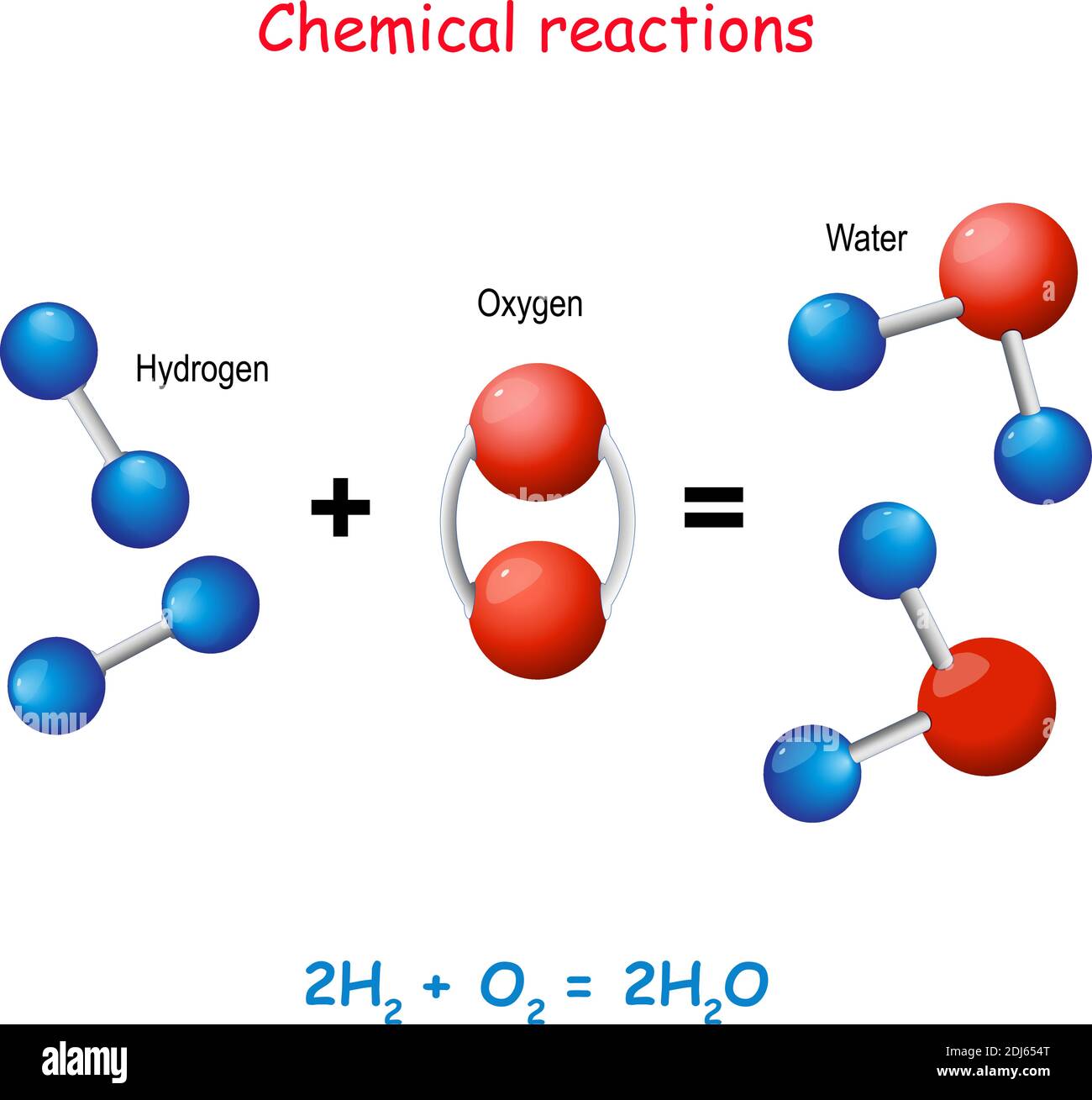
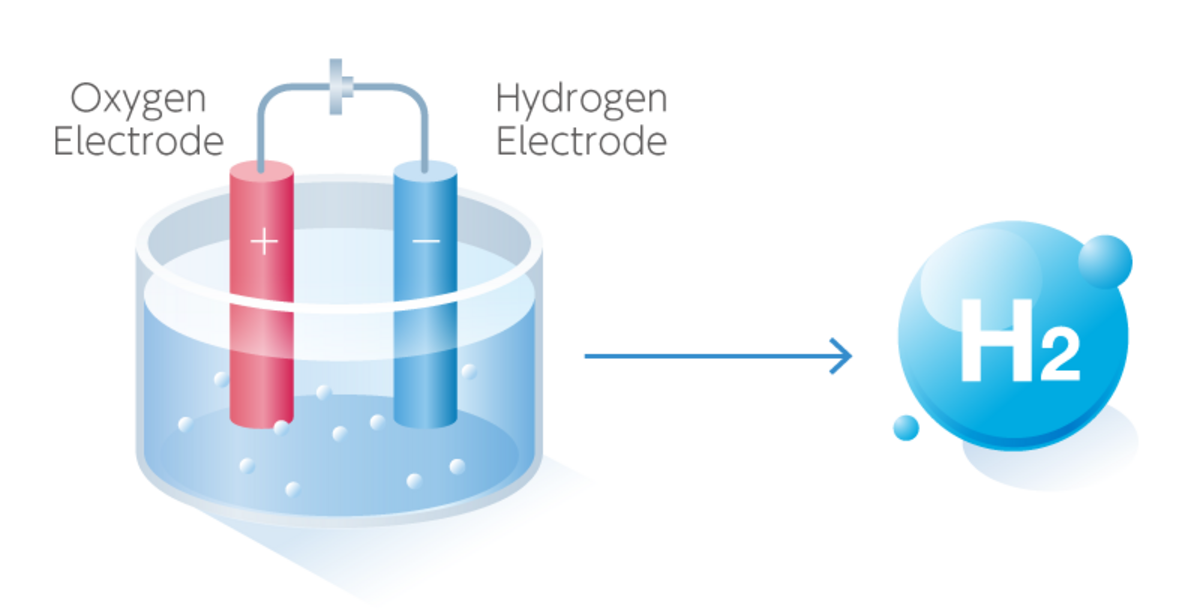
Electrolysis is a well-established technique. It uses electricity to break water (H2O) into its constituent elements: hydrogen and oxygen.
An electrolytic cell, composed of an anode and a cathode submerged in water, conducts electricity. When current flows, water molecules are split, releasing hydrogen gas at the cathode and oxygen at the anode.
The efficiency of electrolysis is impacted by electrode materials, electrolyte type, and applied voltage. Current research focuses on optimizing these factors to maximize hydrogen production and minimize energy consumption.
Challenges with Electrolysis
High energy demands remain a significant obstacle. The process needs to be more energy-efficient to be commercially viable for hydrogen-enriched water production.
Electrode degradation and the need for rare or expensive materials also pose challenges. Finding durable, cost-effective alternatives is a key area of investigation.
Hydrogen Gas Infusion: Direct Dissolution
Directly bubbling hydrogen gas into water is another approach. It involves introducing pressurized H2 gas into water under controlled conditions to promote dissolution.
The amount of hydrogen that dissolves is dependent on temperature, pressure, and the duration of exposure. Higher pressure and lower temperature generally lead to greater hydrogen concentration.
Specialized equipment, like hydrogen generators and pressure vessels, are required. This method offers a more direct route compared to electrolysis.
Limitations of Gas Infusion
The solubility of hydrogen in water is relatively low. This limits the amount of hydrogen that can be dissolved at standard conditions.
Maintaining the dissolved hydrogen concentration over time is difficult. Hydrogen gas tends to escape from the water unless it is contained in a sealed, pressurized environment.
Chemical Reactions: Hydrogen-Releasing Compounds
Certain chemical compounds react with water to release hydrogen gas. These reactions offer an alternative way to introduce hydrogen in situ.
Metal hydrides, for example, react with water to produce hydrogen and a metal hydroxide. This approach allows for controlled release of hydrogen on demand.
The reaction rate can be influenced by the type of metal hydride and the presence of catalysts. Careful selection of the compound is crucial for safety and efficacy.
Concerns with Chemical Reactions
The byproducts of the reaction need to be considered. The resulting metal hydroxides must be safely handled and disposed of.
Controlling the reaction rate is essential. Too rapid a release of hydrogen could be dangerous, while too slow a release might be ineffective.
Nanomaterials: Enhancing Hydrogen Dissolution
Emerging research explores the use of nanomaterials to enhance hydrogen dissolution. Nanoparticles, such as metal nanoparticles or carbon nanotubes, can act as carriers for hydrogen.
These nanomaterials can increase the surface area available for hydrogen adsorption. This facilitates the transfer of hydrogen from the gas phase into the liquid phase.
Functionalizing the surface of nanomaterials with specific chemical groups can further improve hydrogen uptake. This is a promising area for advanced hydrogen-enriched water production.
Challenges for Nanomaterial Application
The long-term stability and safety of nanomaterials in water need to be thoroughly evaluated. Potential toxicity concerns must be addressed before widespread use.
The cost-effectiveness of manufacturing and implementing these nanomaterials needs to be considered. Scalability is a critical factor for practical application.
Current Research and Future Directions
Scientists are focusing on improving the efficiency and stability of existing methods. They are also exploring novel approaches for hydrogen incorporation into water.
Developing advanced materials and optimizing reaction conditions are key priorities. The goal is to achieve high hydrogen concentrations while maintaining safety and cost-effectiveness.
Ongoing research includes investigation of photocatalytic water splitting and sonochemical methods. These innovative approaches hold potential for generating hydrogen directly in water using sunlight or sound waves.
The development of reliable and scalable methods for adding hydrogen to water is critical. It paves the way for advancements in hydrogen-based energy and potential therapeutic applications. Further research and development are essential to unlock the full potential of hydrogen-enriched water.