How Does An Atom Of Aluminum Become An Ion
The invisible world of atoms dictates the properties of everything we see and touch. At the heart of countless materials, aluminum is a common element, known for its lightweight strength and resistance to corrosion. But what happens when a neutral aluminum atom transforms into an electrically charged ion? The answer lies within the intricate dance of electrons and energy.
This article will delve into the process by which an aluminum atom loses electrons to become an ion. Understanding this transformation is crucial because it underpins a vast array of chemical reactions and industrial processes. This includes everything from the creation of aluminum oxide (the protective layer on aluminum) to the electrochemical processes in batteries.
The Atomic Structure of Aluminum
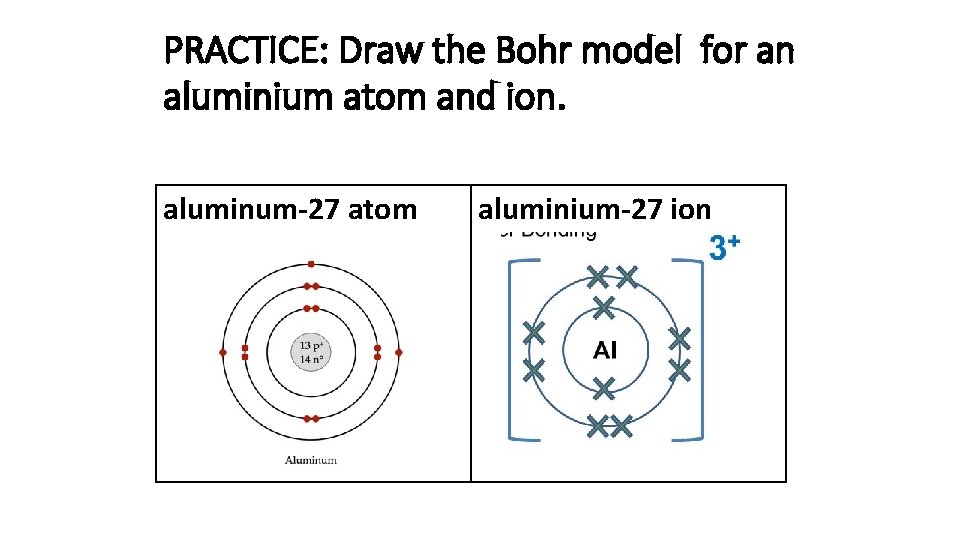
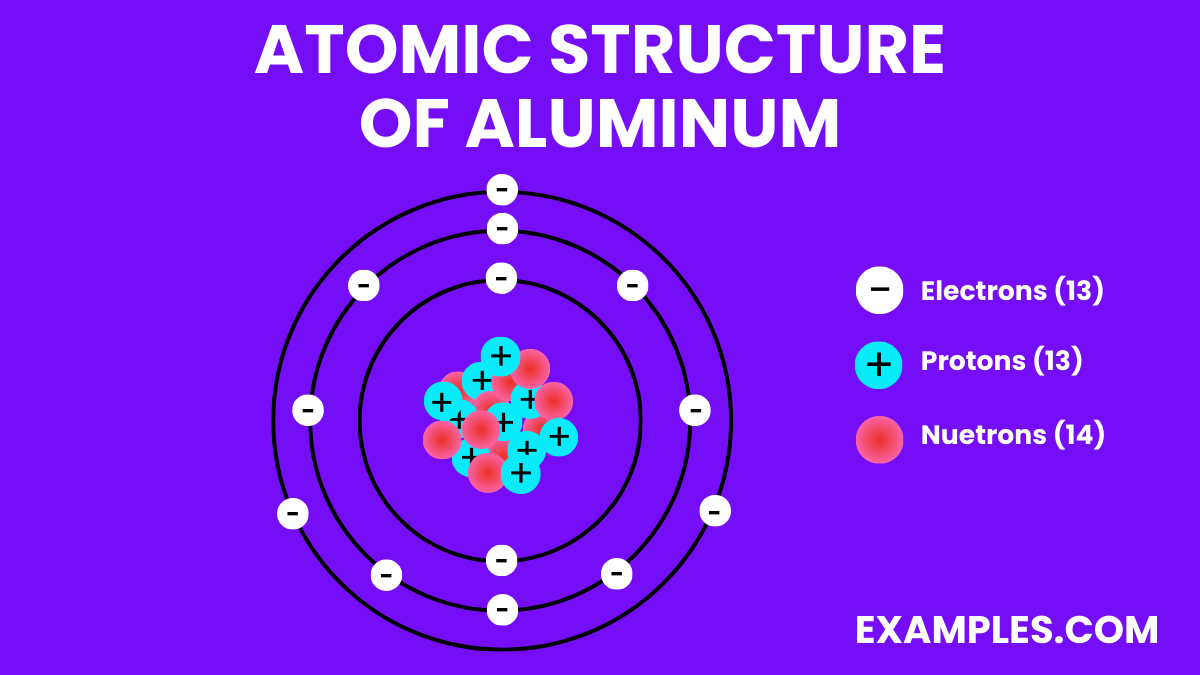
To understand ionization, we must first explore the fundamental structure of an aluminum atom. An aluminum atom possesses 13 protons within its nucleus. These protons give it an atomic number of 13, definitively identifying it as aluminum.
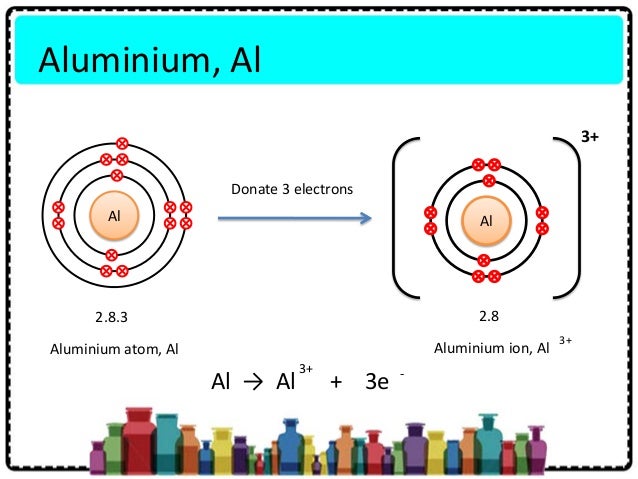
Surrounding the nucleus are 13 electrons, arranged in specific energy levels or shells. The first shell, closest to the nucleus, can hold up to two electrons. The second shell holds up to eight, and the third shell contains the remaining three electrons.
These three electrons in the outermost shell, known as valence electrons, are crucial for chemical bonding and, therefore, ionization. These are the electrons most likely to be involved in interactions with other atoms.
The Path to Ionization: Losing Electrons
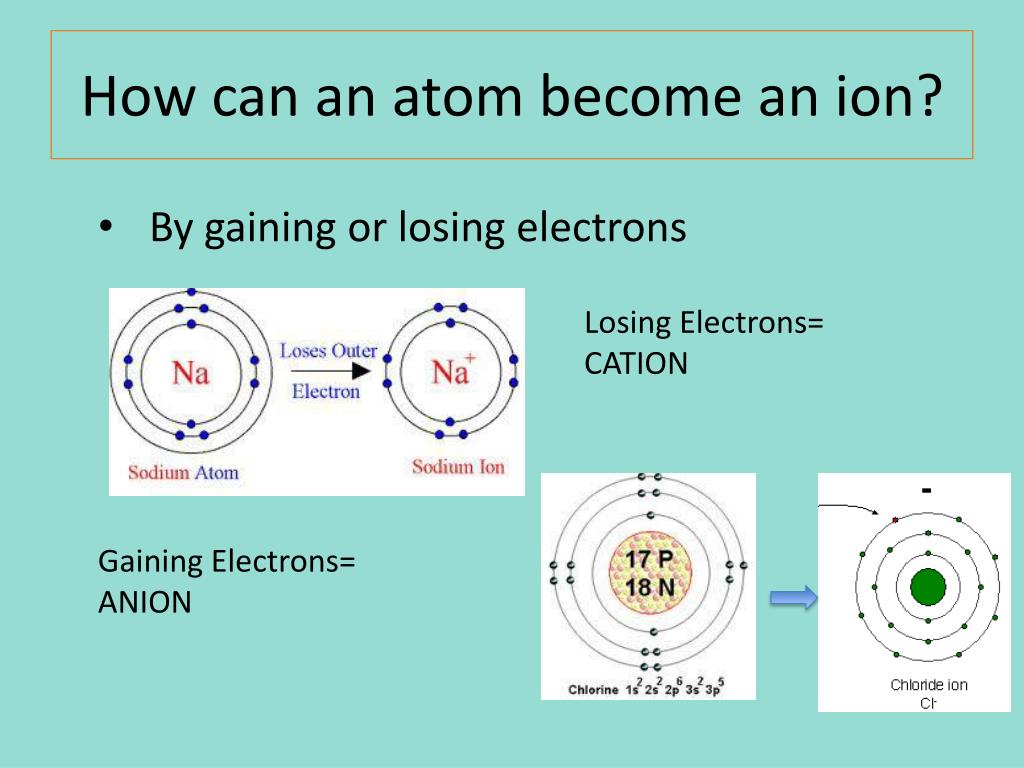
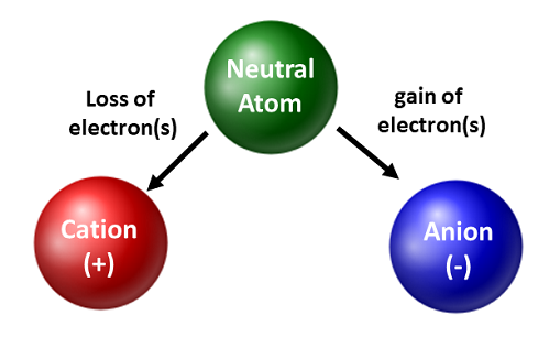
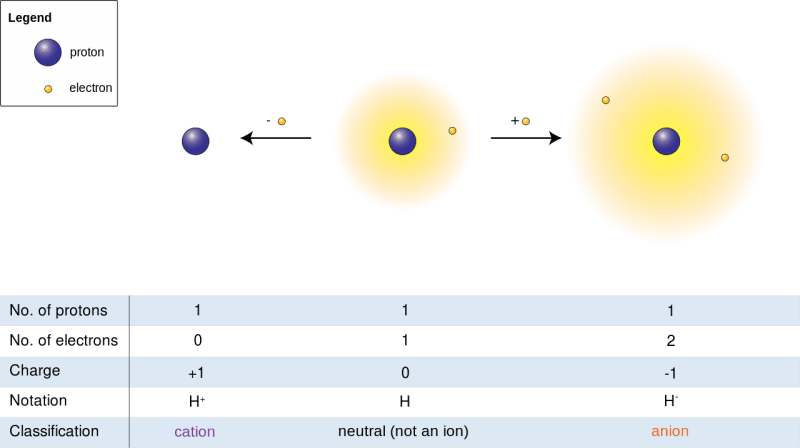
An atom is electrically neutral when the number of protons (positive charge) equals the number of electrons (negative charge). Ionization occurs when an atom gains or loses electrons, disrupting this balance.
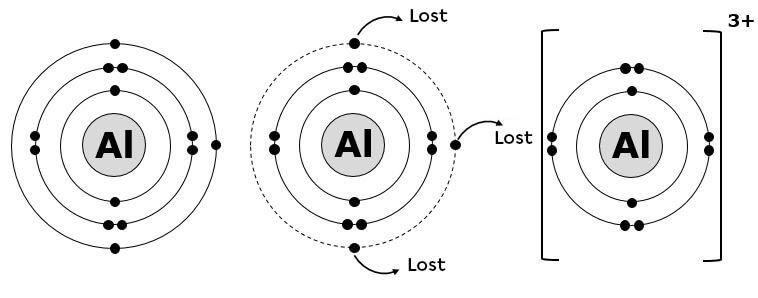
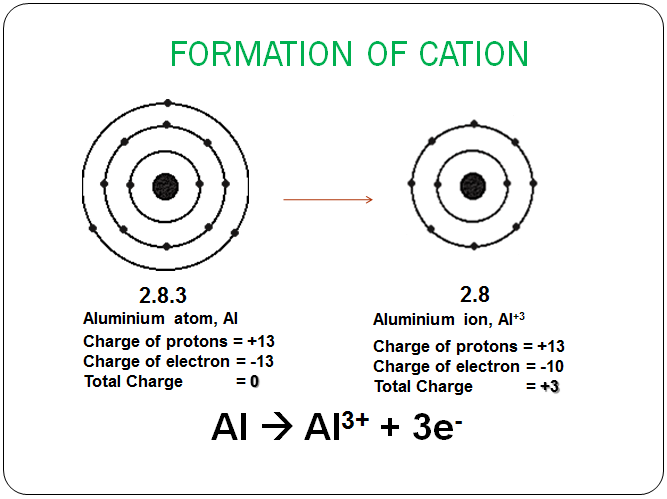
In the case of aluminum, ionization typically involves the loss of its three valence electrons. When an aluminum atom loses these electrons, it becomes a positively charged ion, specifically an aluminum cation (Al3+).
The driving force behind this loss is the pursuit of stability. By losing its three valence electrons, aluminum achieves an electron configuration similar to that of the noble gas neon. This configuration, with a full outer shell, is energetically favorable.
Energy and Ionization Potential
Removing electrons from an atom requires energy. This energy, known as the ionization energy or ionization potential, is specific to each element and each electron. The ionization energy of an element can be found in numerous scientific publications and databases.
Aluminum has three ionization energies corresponding to the removal of its three valence electrons. The first ionization energy (removing one electron) is the lowest. Successively removing the second and third electrons requires significantly more energy because each removal increases the positive charge and the attraction between the nucleus and the remaining electrons.
The values for the first, second, and third ionization energies of aluminum are readily available in chemistry textbooks and online databases. These values are determined experimentally and provide a quantitative measure of the energy required for ionization.
How Ionization Occurs in Practice
The ionization of aluminum can occur through various mechanisms, often involving chemical reactions. A common example is the formation of aluminum oxide (Al2O3), the protective layer that forms on aluminum when exposed to air.
In this process, aluminum atoms react with oxygen atoms. Oxygen, being highly electronegative, readily accepts electrons. Each oxygen atom accepts two electrons, while each aluminum atom loses three.
This transfer of electrons creates positively charged aluminum ions (Al3+) and negatively charged oxygen ions (O2-). These ions then attract each other, forming the ionic compound aluminum oxide.
Electrochemical Processes
Ionization also plays a central role in electrochemical processes, such as those found in batteries and electrolytic cells. The Hall-Héroult process, used for the industrial production of aluminum, is a prime example.
In this process, aluminum oxide is dissolved in molten cryolite, and an electric current is passed through the mixture. At the cathode, aluminum ions (Al3+) gain three electrons to form neutral aluminum atoms.
This process demonstrates the reverse of ionization, where ions gain electrons to become neutral atoms. The energy required for this reduction process is provided by the applied electric current. The efficiency of this process is critically dependent on controlling the ionization and reduction reactions.
"The understanding of ionization energies is crucial for predicting the behavior of elements in chemical reactions." - Dr. Emily Carter, Princeton University
Environmental and Biological Considerations
The presence of aluminum ions in the environment and in biological systems is a topic of ongoing research. While aluminum is abundant in the Earth's crust, its ionic form can be more mobile and potentially bioavailable.
High concentrations of aluminum ions in acidic soils can be toxic to plants, inhibiting root growth and nutrient uptake. In humans, there are concerns about the potential role of aluminum ions in neurodegenerative diseases, although the evidence remains inconclusive.
Further research is needed to fully understand the complex interactions of aluminum ions with biological systems and to develop strategies for mitigating potential risks. The bioavailability of aluminum depends heavily on its chemical form and the environmental conditions.
The Future of Aluminum Ion Research
The study of aluminum ions continues to be an active area of research, with applications ranging from materials science to medicine. Scientists are exploring new ways to manipulate the ionization state of aluminum for various purposes.
One area of focus is the development of new aluminum-based batteries. These batteries could offer high energy density and improved safety compared to conventional lithium-ion batteries.
By gaining a deeper understanding of the factors that govern the ionization of aluminum, researchers hope to unlock its full potential for technological innovation and address environmental challenges. A better understanding of the chemical kinetics is also vital.
In conclusion, the transformation of an aluminum atom into an ion is a fundamental process driven by the pursuit of stability and governed by the laws of quantum mechanics. This process is essential for understanding the chemical behavior of aluminum and its diverse applications in the modern world.