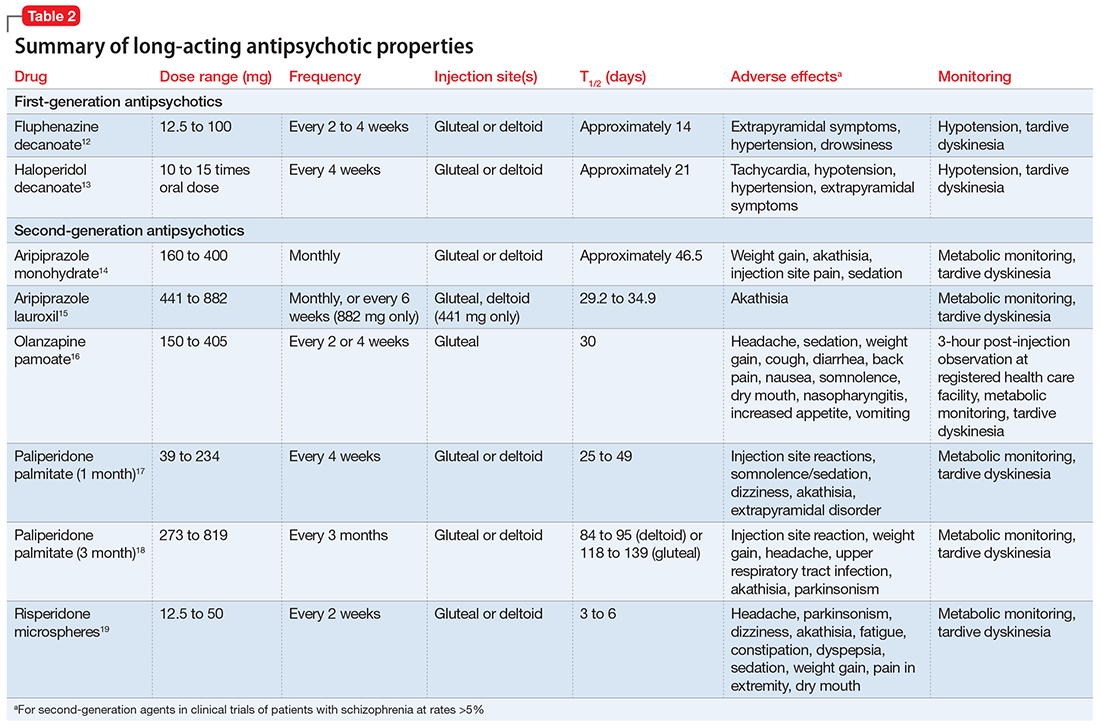
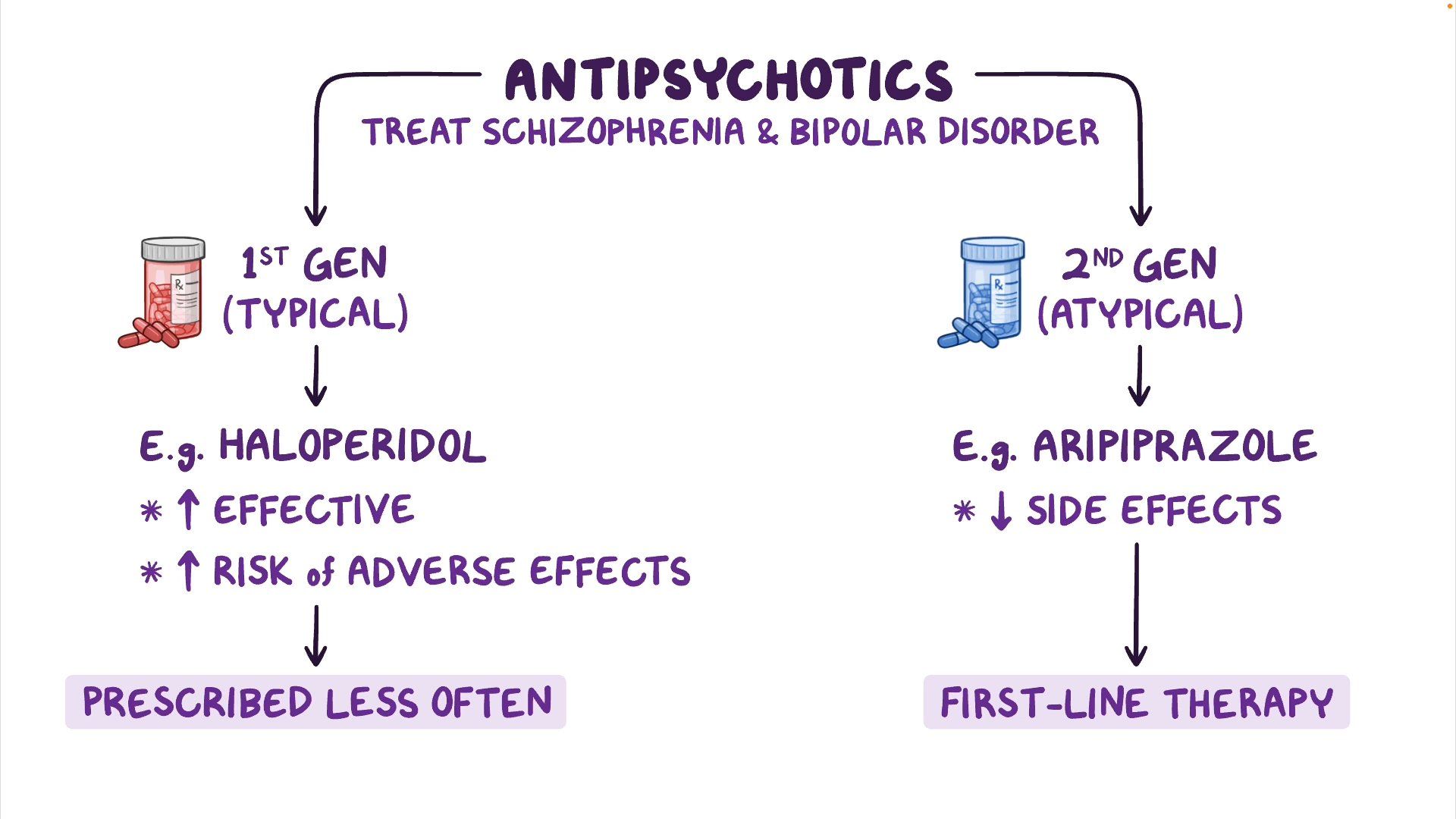
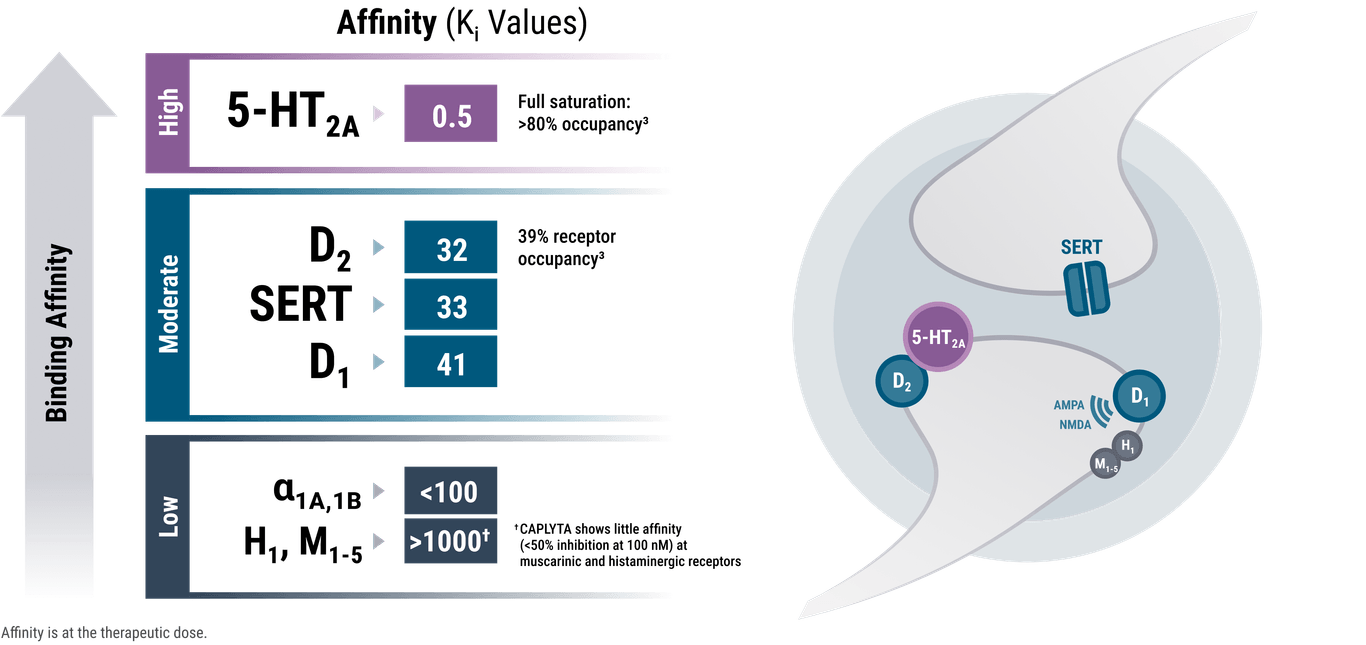How Is Caplyta Different From Other Antipsychotics

The landscape of mental health treatment is constantly evolving, demanding more targeted and effective medications for serious conditions like schizophrenia and bipolar disorder. Antipsychotic drugs, a cornerstone of treatment, have historically been associated with significant side effects, prompting an ongoing search for medications with improved tolerability and efficacy. Among the newer generation of antipsychotics, Caplyta (lumateperone) stands out, offering a potentially distinct mechanism of action and side effect profile compared to its predecessors.
This article delves into the unique properties of Caplyta, exploring how it differs from other antipsychotics in terms of its pharmacological profile, clinical efficacy, and adverse effect profile. We will examine the scientific evidence supporting its use, compare it to established treatments, and consider its potential place in the future of mental health care. This analysis aims to provide a comprehensive understanding of Caplyta's potential benefits and limitations, ultimately informing clinicians and patients alike.
A Novel Mechanism of Action
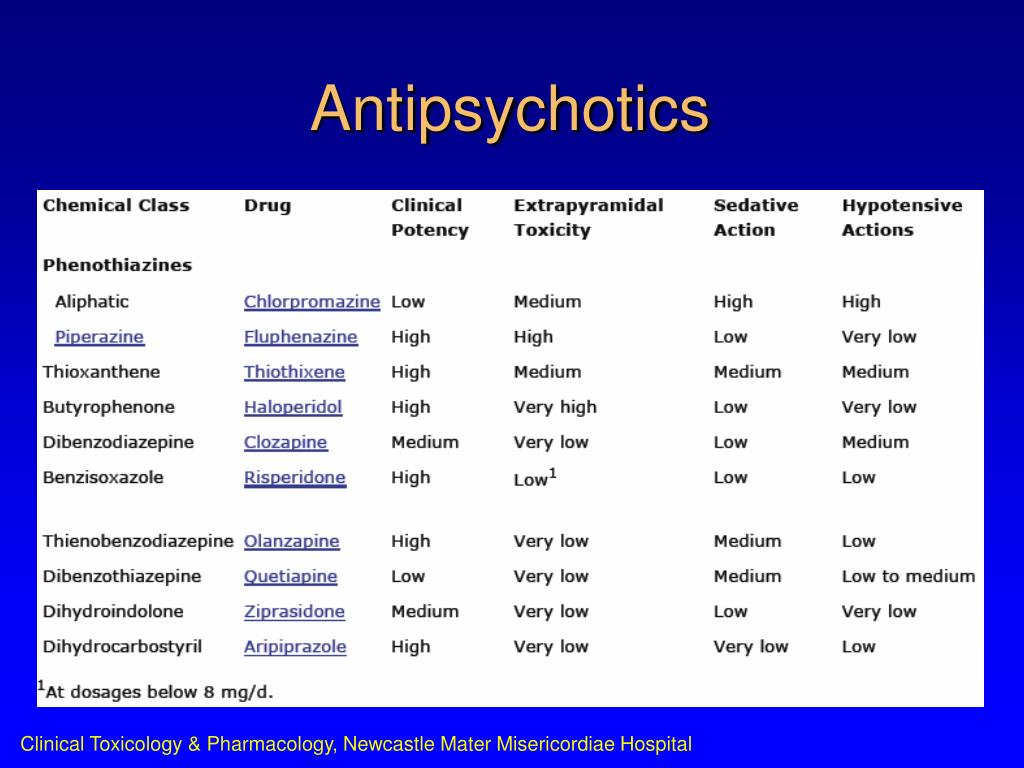
Traditional antipsychotics primarily act by blocking dopamine D2 receptors in the brain, which can effectively reduce psychotic symptoms but often leads to side effects like movement disorders. Caplyta, however, employs a more nuanced approach.
Caplyta's primary mechanism involves modulating dopamine, serotonin, and glutamate neurotransmitter systems. Its action is primarily as a selective serotonin 5-HT2A receptor antagonist, but it also acts as a dopamine D1 receptor modulator, and possesses low affinity for D2 receptors. This combination is thought to contribute to its potential for fewer motor-related side effects.
The unique modulation of these neurotransmitter systems is theorized to produce a more targeted therapeutic effect. Unlike many older antipsychotics, its D2 receptor occupancy is low, potentially explaining its lower risk of extrapyramidal symptoms (EPS), a common side effect of first-generation antipsychotics and some second-generation antipsychotics.
Clinical Efficacy and Indications
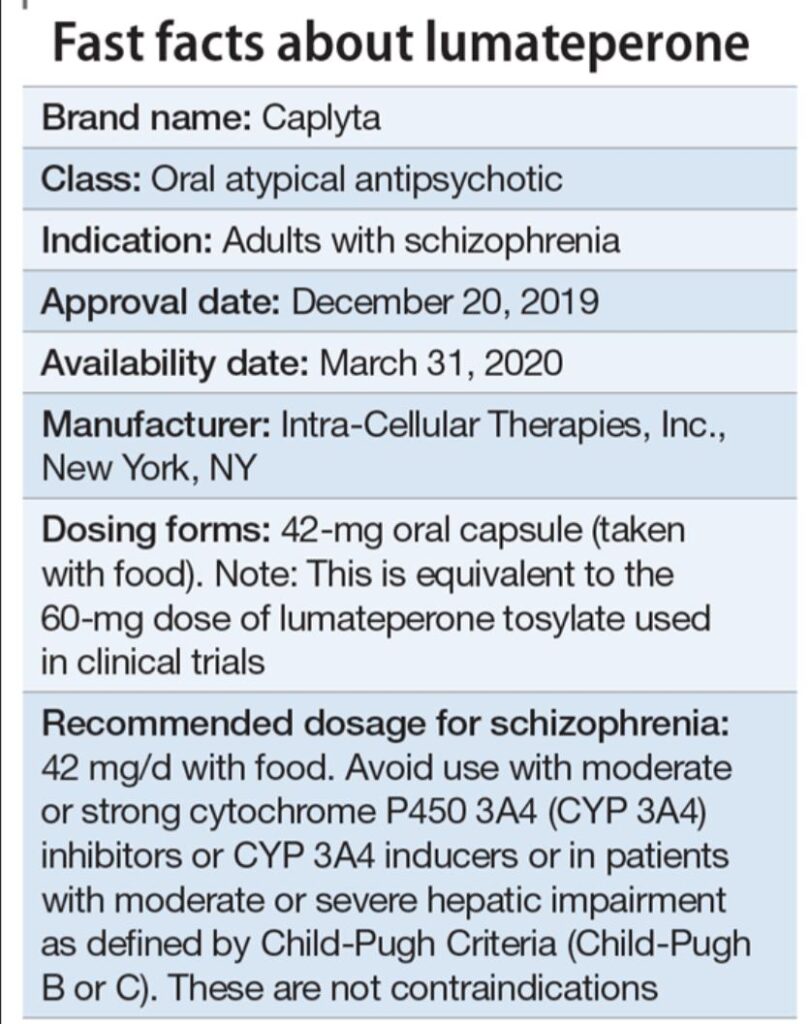
Caplyta is approved by the FDA for the treatment of schizophrenia in adults and for depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults. Clinical trials have demonstrated its efficacy in reducing both positive and negative symptoms of schizophrenia.
Studies have shown that Caplyta significantly improves scores on standardized scales that measure the severity of schizophrenia symptoms. Similarly, in patients with bipolar depression, clinical trials have demonstrated significant reductions in depressive symptoms compared to placebo.
The efficacy data support Caplyta as a viable option for individuals struggling with these challenging mental health conditions. It provides clinicians with another tool in their arsenal to help patients achieve symptom control and improve their quality of life.
Comparing Side Effect Profiles
One of the most significant differences between Caplyta and many other antipsychotics lies in its side effect profile. While all antipsychotics carry the potential for adverse effects, Caplyta appears to have a more favorable profile compared to some older medications.
Clinical trials have indicated a lower incidence of EPS, such as tremors, muscle stiffness, and restlessness, compared to some other antipsychotics. This is attributed to its unique mechanism of action that minimizes D2 receptor blockade. Other common side effects include somnolence (drowsiness) and dry mouth.
Metabolic side effects, such as weight gain, increased cholesterol, and elevated blood sugar, are also a concern with many antipsychotics. While Caplyta can cause metabolic changes, studies suggest that the incidence of significant weight gain and metabolic disturbances may be lower compared to some other second-generation antipsychotics, though careful monitoring is still warranted. Clinicians must carefully consider each patient's individual risk factors when selecting an antipsychotic.
Perspectives From Professionals
Mental health professionals acknowledge the potential benefits of Caplyta but also emphasize the importance of individualized treatment approaches. Dr. Emily Carter, a psychiatrist specializing in psychotic disorders, states, "Caplyta offers a valuable alternative for patients who haven't responded well to other antipsychotics or who have experienced intolerable side effects."
However, Dr. Carter also cautions, "It's crucial to remember that every patient is different, and what works for one person may not work for another. A thorough evaluation and careful monitoring are essential when initiating any new medication."
Professor David Miller, a leading researcher in psychopharmacology, emphasizes the need for more long-term data on Caplyta's efficacy and safety. "While the initial clinical trial results are promising, we need more real-world evidence to fully understand its long-term effects and how it compares to other treatment options over extended periods."
The Future of Antipsychotic Treatment
Caplyta represents an important step forward in the development of more targeted and tolerable antipsychotic medications. Its unique mechanism of action and potentially improved side effect profile offer hope for individuals struggling with schizophrenia and bipolar depression.
Ongoing research is exploring the potential of Caplyta in other psychiatric conditions, as well as its use in combination with other treatments. As we continue to gain a deeper understanding of the complex neurobiology of mental illness, we can expect to see further advancements in the development of more effective and personalized treatment strategies.
Ultimately, the goal is to provide individuals with mental health conditions with the tools they need to live fulfilling and productive lives. Caplyta, with its novel approach, contributes to this ongoing effort to refine and improve the treatment of severe mental illnesses.