How Many Energy Levels Are There

Imagine a hummingbird, wings a blur, flitting between vibrant blossoms. Each sip of nectar is a burst of energy, a tiny transaction governed by the invisible rules of the quantum world. But what if that hummingbird was an electron, and those blossoms were energy levels, the very foundations upon which matter, and indeed, everything we know, is built?
The question of how many energy levels exist isn’t as simple as counting petals on a flower. At its heart, it delves into the fundamental nature of quantum mechanics. While the number of energy levels within a single atom is theoretically infinite, the practical reality is far more nuanced, shaped by factors like the atom's complexity and the forces acting upon it.
Our journey to understanding energy levels begins with a giant leap backward, to the early 20th century, when classical physics began to crumble under the weight of experimental evidence. Thinkers like Max Planck and Albert Einstein dared to suggest that energy wasn't continuous, but rather came in discrete packets, or quanta.
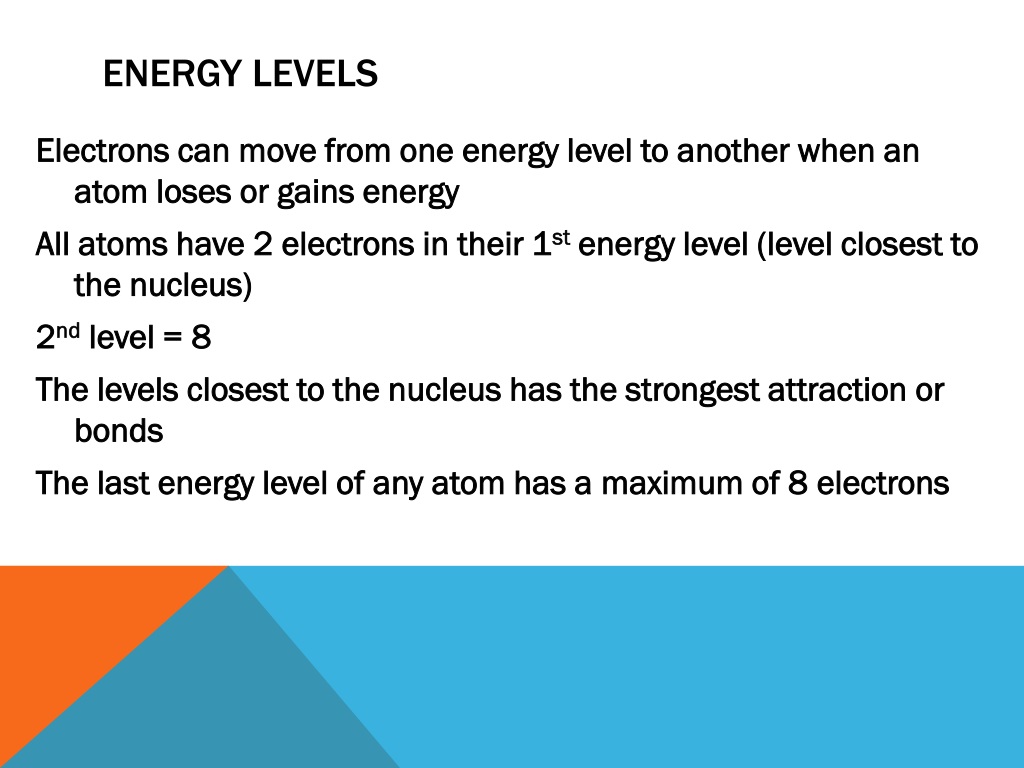
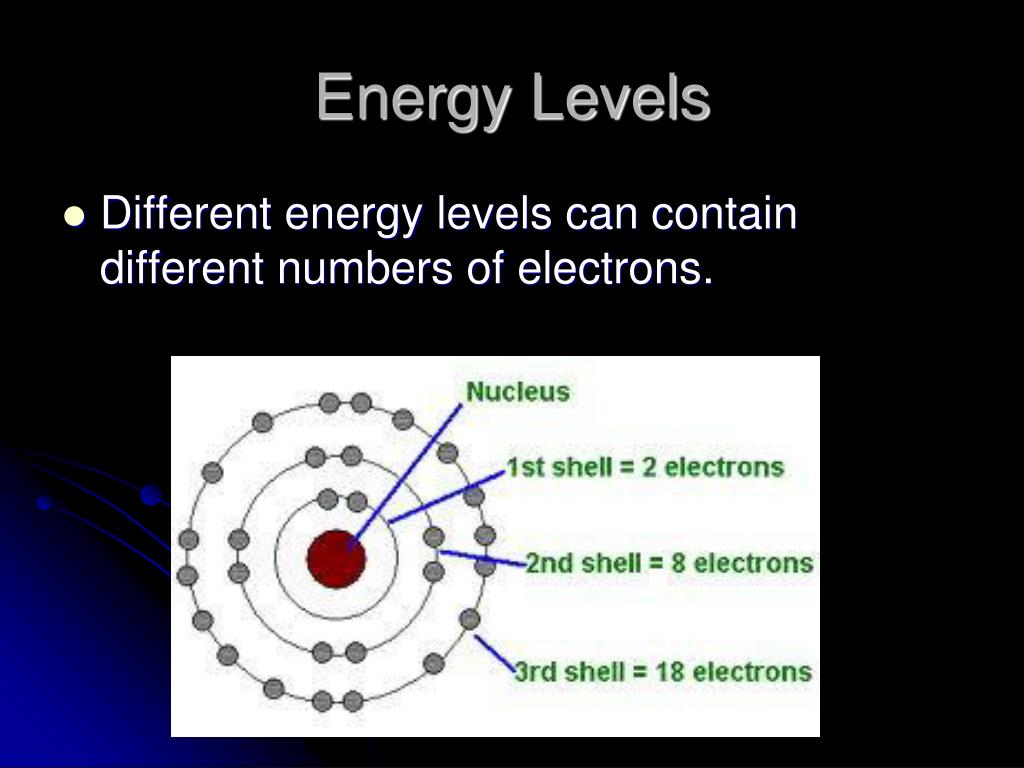
This revolutionary idea was solidified by Niels Bohr, who applied it to the structure of the atom. He proposed that electrons orbit the nucleus in specific, quantized energy levels, much like planets orbiting a star at fixed distances.
Bohr's model, while ultimately superseded by more sophisticated theories, was a monumental step forward. It explained why atoms only absorb and emit light at specific wavelengths, a phenomenon observed in atomic spectra. When an electron jumps from a higher energy level to a lower one, it releases energy in the form of a photon, a particle of light with a specific wavelength.
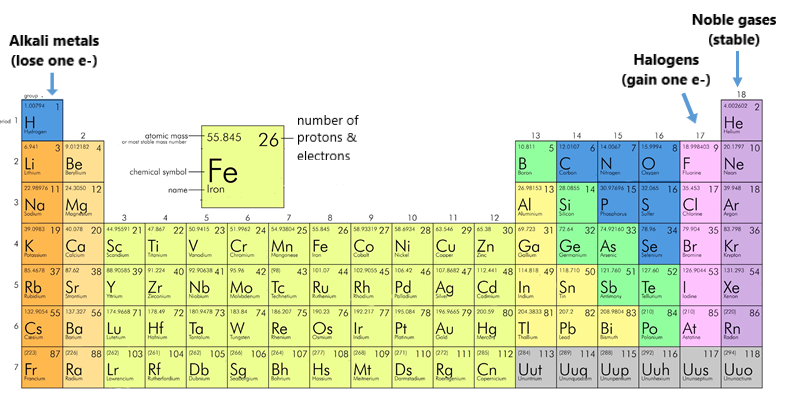
Conversely, an electron can absorb a photon of the correct wavelength and jump to a higher energy level. Each element possesses a unique set of energy levels, leading to distinctive spectral fingerprints.
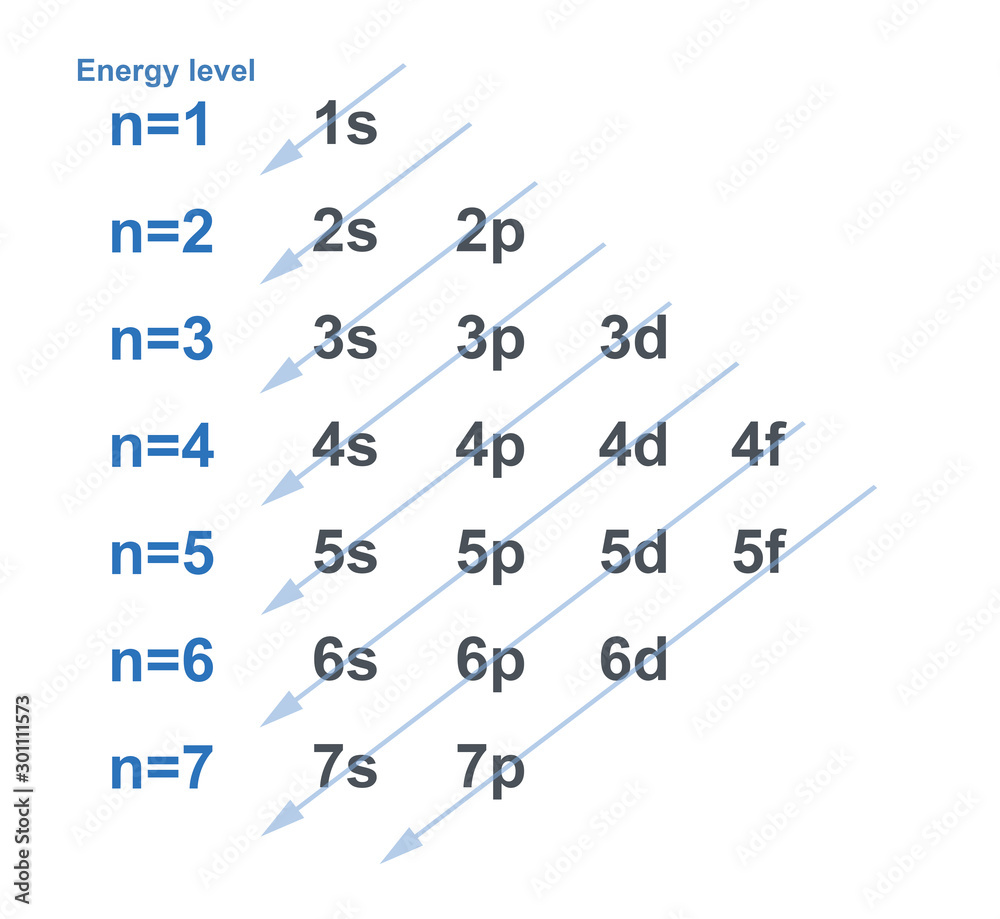
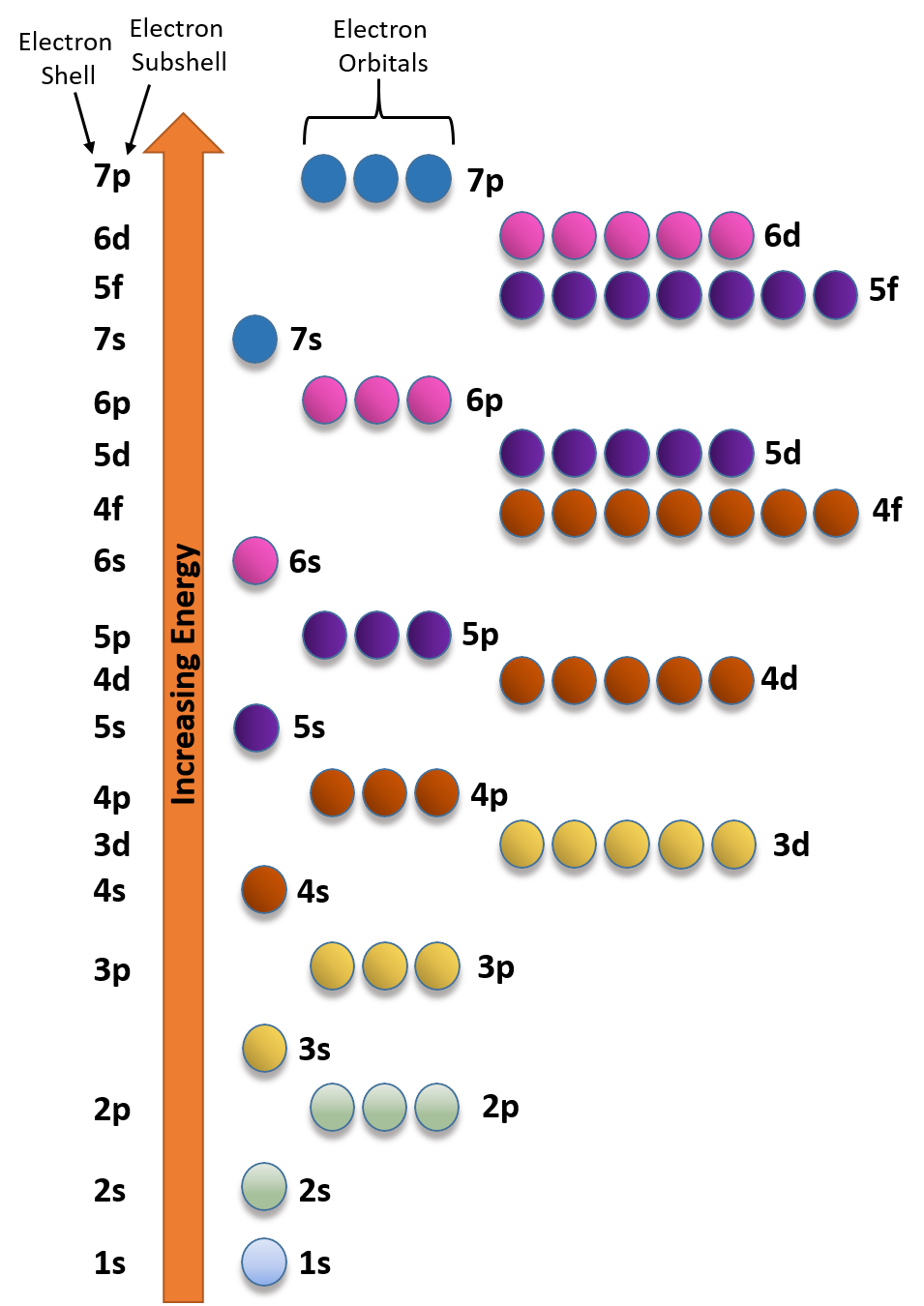
The concept of energy levels is elegantly described by quantum mechanics, which replaces Bohr's planetary orbits with probability distributions called atomic orbitals. These orbitals represent regions of space where an electron is most likely to be found, each corresponding to a specific energy level.
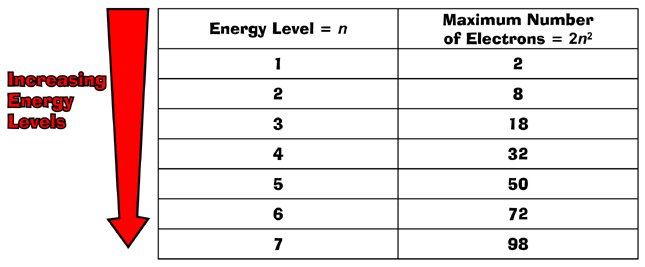
According to the principles of quantum mechanics, an isolated atom possesses an infinite number of energy levels. As energy increases, the spacing between these levels decreases, approaching a continuum at very high energies.
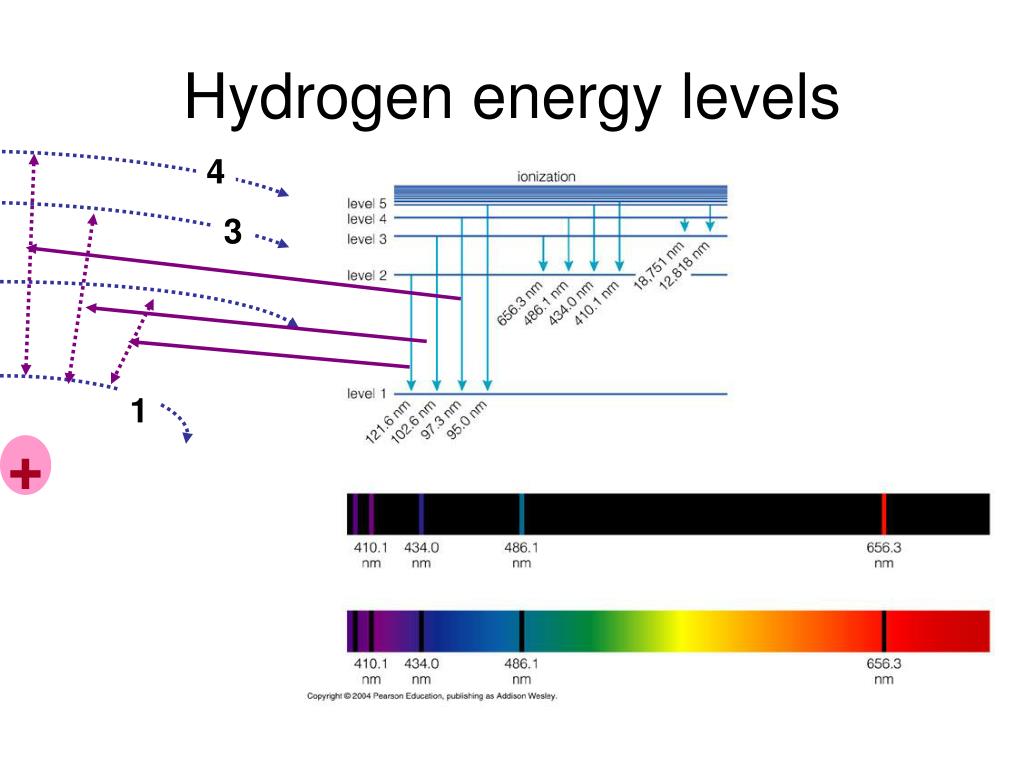
However, the practical reality is that we can only observe a finite number of these levels. For instance, hydrogen, the simplest atom, has an infinite number of energy levels theoretically.
But when we excite a hydrogen atom in a laboratory, we only observe a finite number of spectral lines, corresponding to transitions between a limited range of energy levels. The higher energy levels are so closely spaced that they become increasingly difficult to distinguish experimentally.
The Influence of External Factors
The number and characteristics of energy levels are not solely determined by the atom itself. External factors like electric and magnetic fields, as well as interactions with neighboring atoms, can significantly alter the energy level structure.
For example, applying a magnetic field to an atom causes energy levels to split into multiple sublevels, a phenomenon known as the Zeeman effect. This splitting is crucial in various applications, including magnetic resonance imaging (MRI) and atomic clocks.
In solids, the interactions between atoms lead to the formation of energy bands, rather than discrete energy levels. These bands dictate the electrical conductivity of materials.
In semiconductors, the gap between the valence band (where electrons reside at low energies) and the conduction band (where electrons can move freely) determines whether the material conducts electricity easily or acts as an insulator. The control and manipulation of these energy bands are at the heart of modern electronics.
Energy Levels in Molecules
The concept of energy levels extends beyond individual atoms to encompass molecules. Molecules possess not only electronic energy levels, similar to atoms, but also vibrational and rotational energy levels.
Vibrational energy levels correspond to the different ways that atoms within a molecule can vibrate relative to each other. Rotational energy levels correspond to the different ways that the molecule can rotate.
These vibrational and rotational energy levels are quantized, meaning that molecules can only vibrate and rotate at specific frequencies. Transitions between these levels give rise to the rich infrared and microwave spectra of molecules, providing valuable information about their structure and dynamics.
Applications of Energy Level Understanding
The knowledge of energy levels is not confined to theoretical physics; it has profound practical applications across a wide range of fields. Spectroscopy, the study of how matter interacts with electromagnetic radiation, relies heavily on the understanding of energy level transitions.
Astronomers use spectroscopy to determine the composition and temperature of stars by analyzing the light they emit. Medical professionals use it in diagnostic tools.
Laser technology is another prime example of the importance of energy levels. Lasers work by stimulating the emission of photons from atoms in a specific energy state, creating a coherent beam of light.
The precise control of energy levels is crucial for achieving efficient and powerful laser operation. From barcode scanners to surgical instruments, lasers have revolutionized numerous industries.
Quantum Computing
One of the most exciting emerging applications of energy level control is in the field of quantum computing. Qubits, the fundamental units of quantum information, can be realized using the energy levels of individual atoms or ions.
By carefully manipulating these energy levels with lasers or microwaves, scientists can perform complex calculations that are beyond the reach of classical computers. Quantum computing has the potential to revolutionize fields like medicine, materials science, and artificial intelligence.
Looking Ahead
The quest to understand and manipulate energy levels is an ongoing endeavor. Scientists are constantly developing new techniques to probe the intricacies of atomic and molecular structure, pushing the boundaries of what is possible.
Advances in ultrafast laser technology allow researchers to study energy level transitions on the femtosecond (10-15 second) timescale, providing unprecedented insights into the dynamics of chemical reactions. Novel materials with tailored energy level structures are being designed for applications in solar energy, optoelectronics, and quantum technologies.
While the question of exactly how many energy levels exist may seem abstract, it touches upon the very essence of reality. The dance of electrons between energy levels governs the behavior of atoms and molecules, shaping the world around us.
As we delve deeper into the quantum realm, we unlock new possibilities for innovation and a greater appreciation for the elegant simplicity underlying the complexity of the universe.








+are+fixed+amounts+of+energy+located+in+a+specific+region+around+the+nucleus..jpg)