Immunogenicity Assessment For Therapeutic Protein Products
Imagine a world where life-saving medications could, unexpectedly, turn against the very people they're meant to heal. This isn't a scene from a dystopian novel, but a real concern in the realm of therapeutic proteins, where the body's immune system can sometimes mistake these beneficial treatments for foreign invaders, triggering an unwanted response.
At the heart of ensuring the safety and efficacy of these vital medicines lies immunogenicity assessment, a rigorous process that predicts, detects, and characterizes how the body might react to a therapeutic protein.
This article delves into the complexities of immunogenicity assessment for therapeutic protein products, exploring its background, significance, and the cutting-edge strategies being employed to safeguard patients and optimize treatment outcomes.
The Rise of Therapeutic Proteins and the Immunogenicity Challenge
Therapeutic proteins, including monoclonal antibodies, enzymes, and cytokines, have revolutionized the treatment of numerous diseases, from cancer and autoimmune disorders to genetic deficiencies.
These complex molecules offer highly targeted therapies with the potential for greater efficacy and fewer side effects than traditional small-molecule drugs.
However, their inherent complexity also presents a unique challenge: the risk of inducing an immune response.
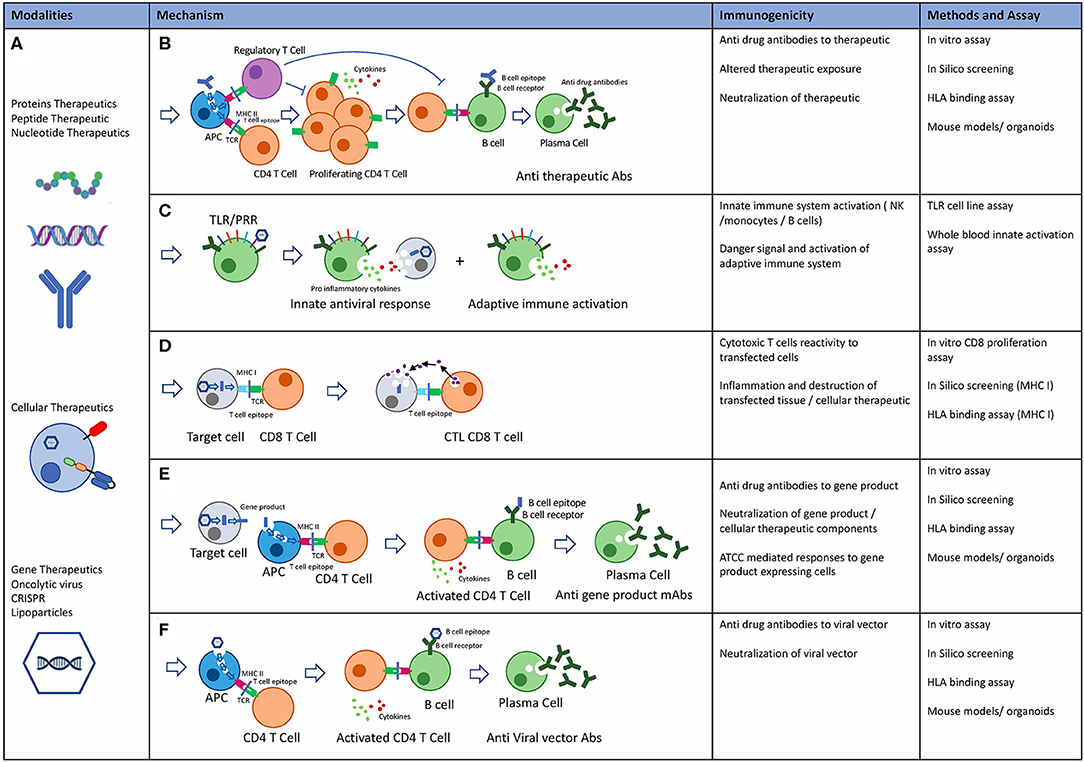
When the body recognizes a therapeutic protein as foreign, it can produce anti-drug antibodies (ADAs).
These ADAs can neutralize the drug's activity, leading to treatment failure, or, in more severe cases, cause adverse events like infusion reactions or even life-threatening autoimmune conditions.
The incidence and severity of immunogenicity can vary significantly depending on factors such as the protein's structure, the patient's genetic background, the route of administration, and the presence of pre-existing immune conditions.
Understanding Immunogenicity Assessment
Immunogenicity assessment is a multi-faceted process designed to minimize the risk of adverse immune responses and ensure that therapeutic proteins remain safe and effective.
It encompasses a range of activities, starting with in silico and in vitro studies during early drug development and continuing through clinical trials and post-market surveillance.
Predictive Assessment: Minimizing Risk from the Start
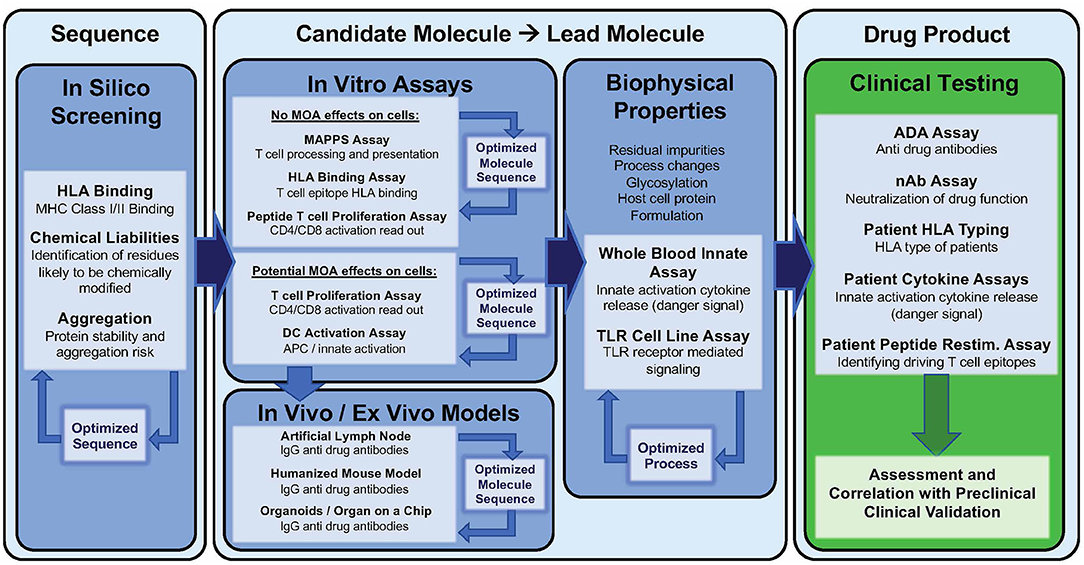
The first step involves predicting the potential immunogenicity of a therapeutic protein based on its amino acid sequence, structure, and potential for aggregation.
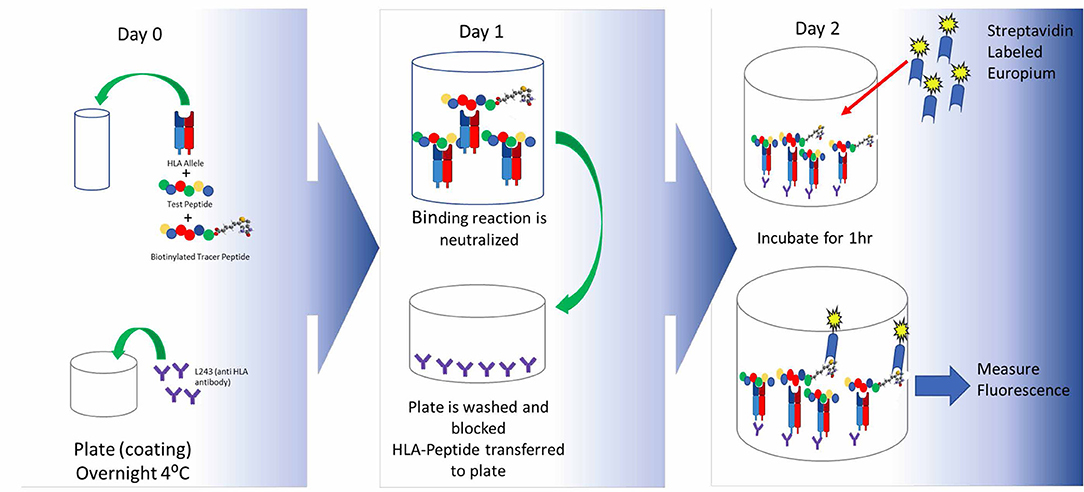
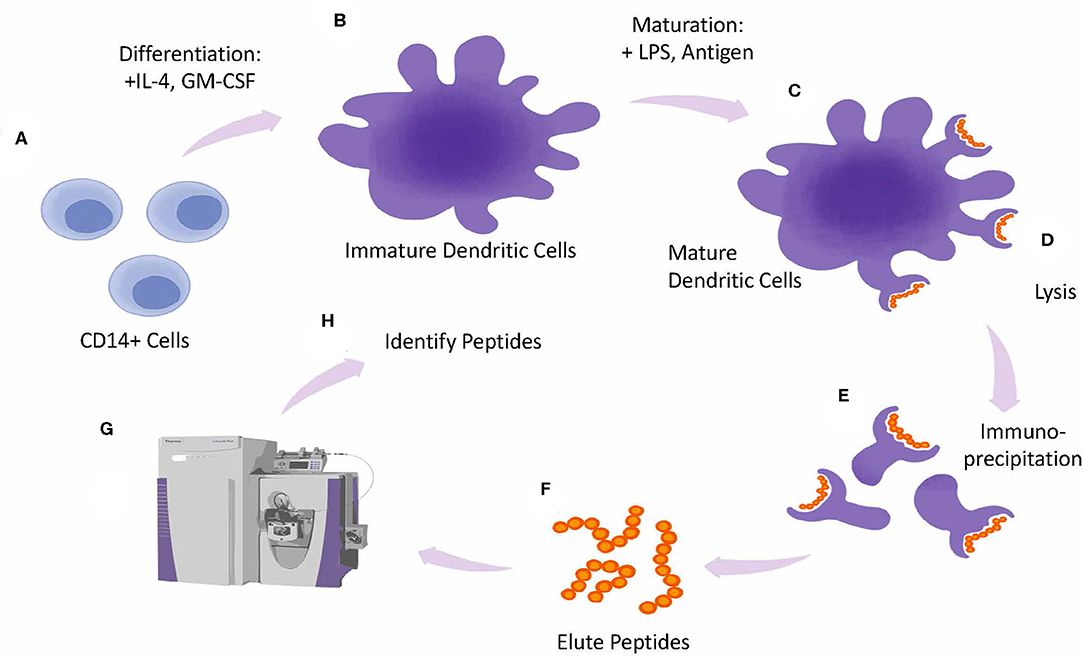
In silico tools are used to identify regions of the protein that are likely to bind to major histocompatibility complex (MHC) molecules, which are key players in initiating an immune response.
In vitro assays, such as T-cell proliferation assays, can then be used to assess the ability of the protein to stimulate immune cells in a controlled laboratory setting.
Detection and Characterization: Monitoring Immune Responses in Patients
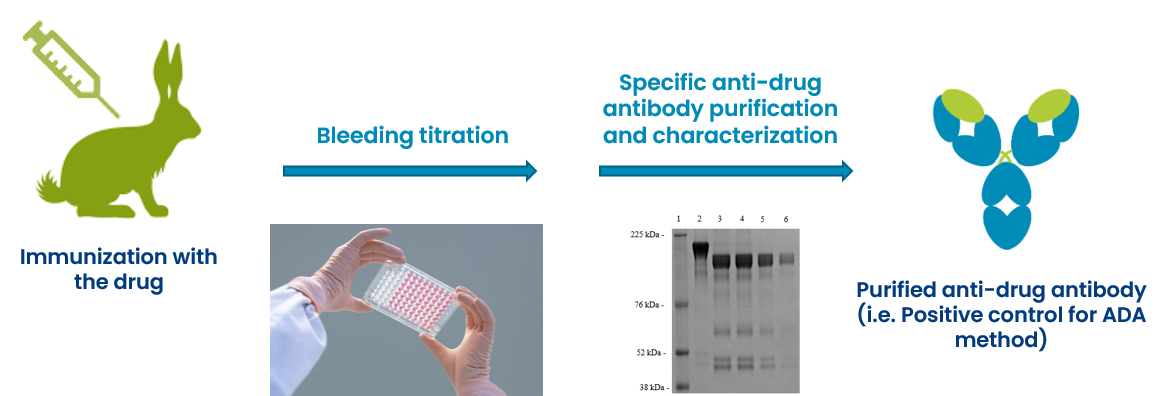
During clinical trials, patients are carefully monitored for the development of ADAs using a variety of analytical methods.
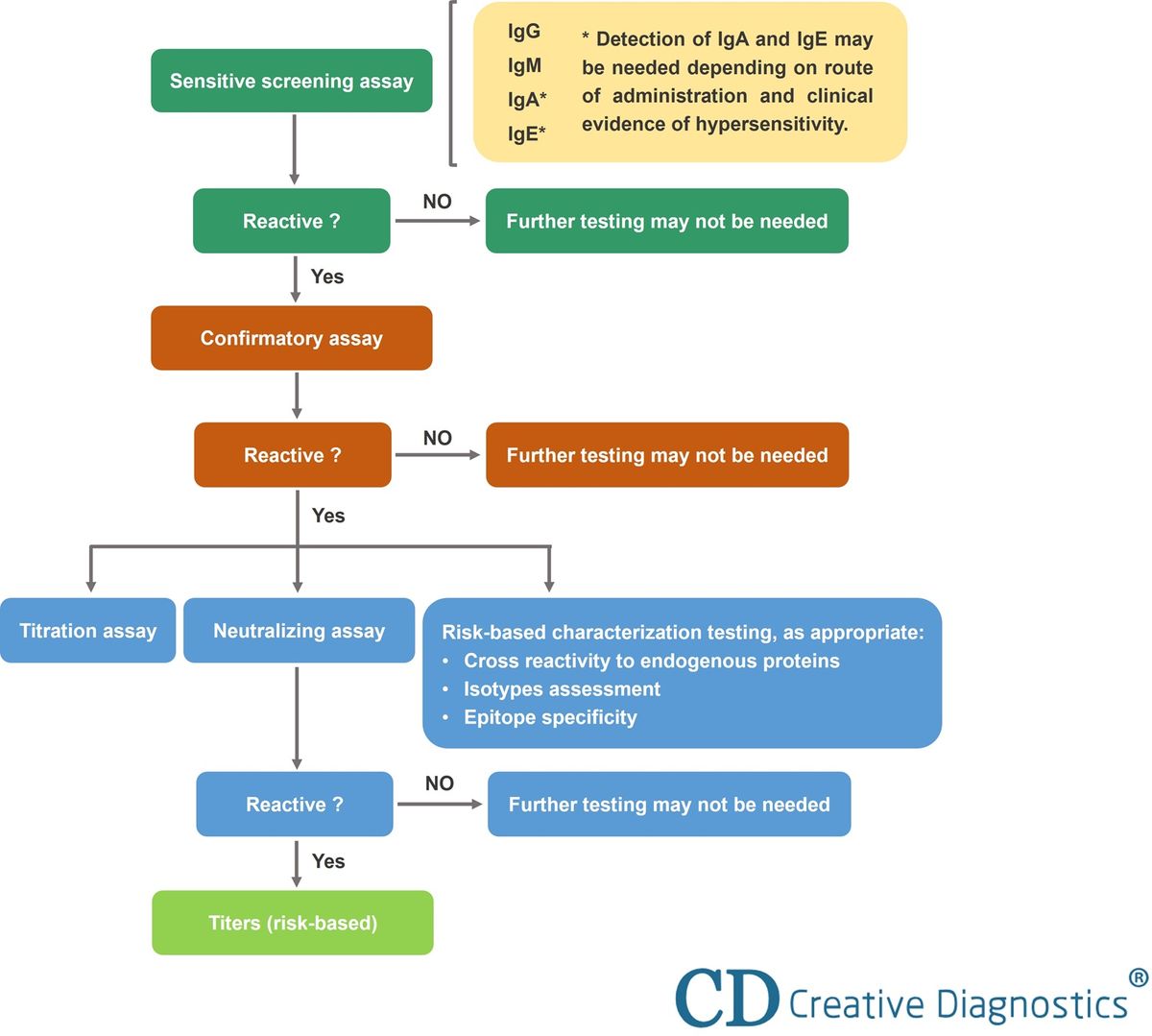
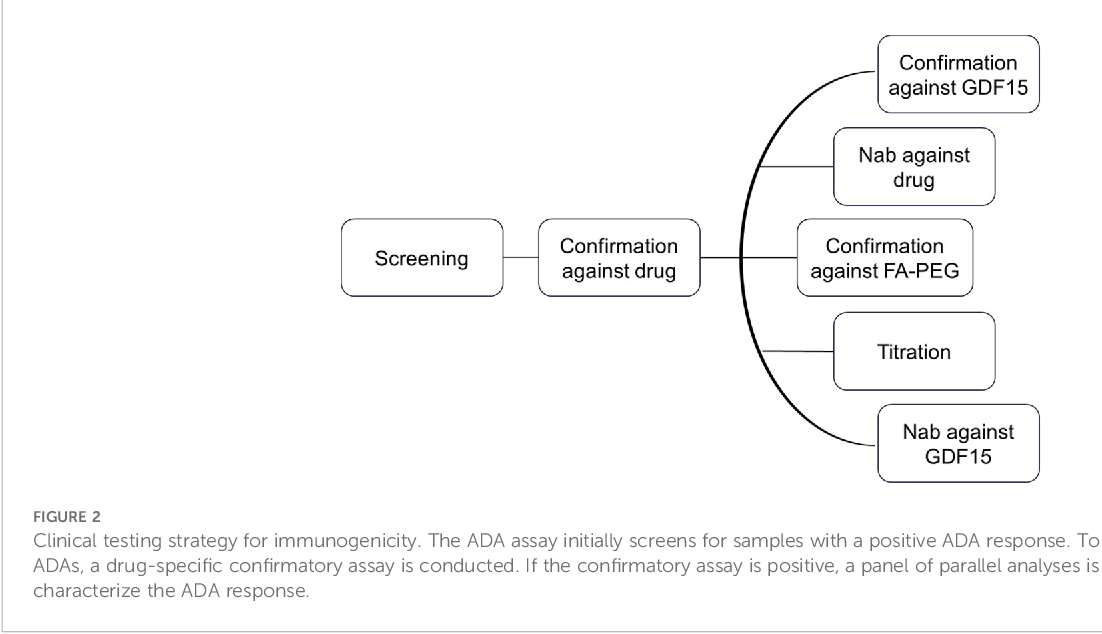
These methods typically involve a tiered approach, starting with a screening assay to detect the presence of ADAs, followed by a confirmatory assay to rule out false positives.
If ADAs are detected, they are further characterized to determine their titer (concentration), isotype (type of antibody), and neutralizing capacity.
"A comprehensive immunogenicity assessment strategy is essential for ensuring the safety and efficacy of therapeutic protein products." - FDA Guidance Document
Advanced Techniques and Emerging Technologies
The field of immunogenicity assessment is constantly evolving, with new technologies and approaches being developed to improve the accuracy and sensitivity of detection methods.
These include:
- Surface Plasmon Resonance (SPR): A label-free technique that can be used to measure the binding affinity of ADAs to the therapeutic protein.
- Cell-Based Assays: More physiologically relevant assays that can mimic the complex interactions between immune cells and the therapeutic protein.
- Mass Spectrometry: A powerful technique that can be used to identify and characterize ADAs at the molecular level.
Factors Influencing Immunogenicity
Several factors can influence the immunogenicity of a therapeutic protein.
Understanding these factors is crucial for designing strategies to minimize the risk of adverse immune responses.
Product-Related Factors
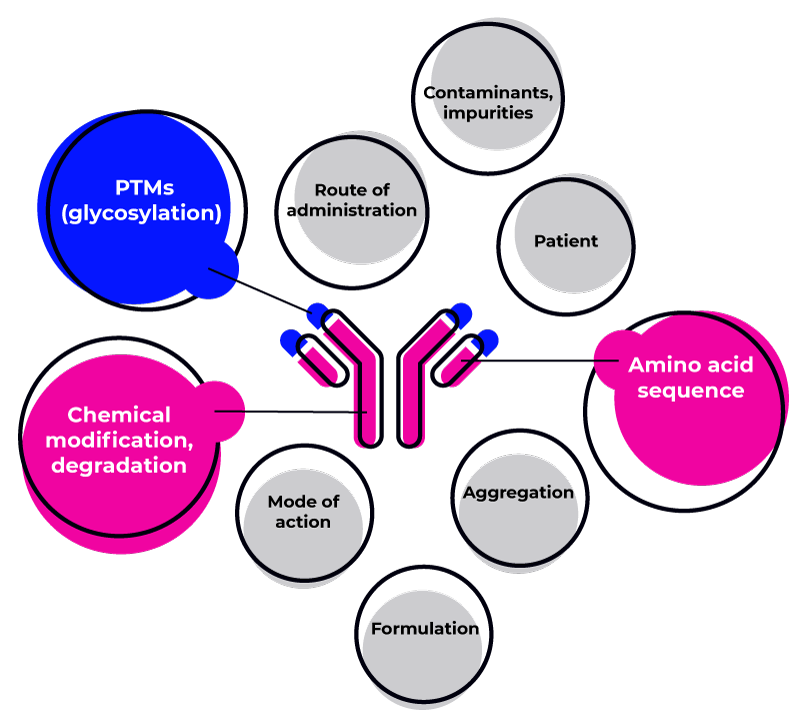
The structure and sequence of the protein itself play a significant role.
Proteins with novel sequences or post-translational modifications are more likely to be recognized as foreign by the immune system.
Impurities introduced during the manufacturing process, such as host cell proteins or aggregates, can also contribute to immunogenicity.
Patient-Related Factors
The patient's genetic background, particularly their HLA type, can influence their susceptibility to developing ADAs.
Patients with pre-existing immune conditions, such as autoimmune diseases, are also at higher risk.
Age, gender, and ethnicity can also play a role.
Treatment-Related Factors
The route of administration can affect immunogenicity.
Subcutaneous injections, for example, are more likely to induce an immune response than intravenous infusions.
The dose and frequency of administration can also influence the development of ADAs.
Strategies to Minimize Immunogenicity
A variety of strategies are being employed to minimize the immunogenicity of therapeutic proteins, including:
- Humanization: Modifying the protein sequence to make it more similar to human proteins.
- Glycosylation Engineering: Altering the glycosylation pattern of the protein to reduce its immunogenicity.
- Formulation Optimization: Developing formulations that minimize protein aggregation and degradation.
- Immunosuppression: Co-administering immunosuppressive drugs to suppress the immune response.
The Regulatory Landscape
Regulatory agencies like the FDA and the EMA have established guidelines for immunogenicity assessment of therapeutic protein products.
These guidelines emphasize the importance of a risk-based approach, with more comprehensive testing required for products with a higher potential for immunogenicity.
Manufacturers are expected to develop and implement robust immunogenicity assessment strategies that address both product-related and patient-related factors.
The Future of Immunogenicity Assessment
The field of immunogenicity assessment is poised for continued innovation, driven by advances in technology and a deeper understanding of the immune system.
Personalized medicine approaches, which take into account individual patient characteristics, are likely to play an increasingly important role in predicting and managing immunogenicity.
Artificial intelligence and machine learning algorithms are also being developed to analyze large datasets and identify patterns that can predict the likelihood of an immune response.
Conclusion
Immunogenicity assessment is a critical component of therapeutic protein development, ensuring that these life-saving medications remain safe and effective for all patients.
By understanding the complexities of the immune response and employing advanced techniques to predict, detect, and characterize ADAs, we can minimize the risk of adverse events and optimize treatment outcomes.
As the field continues to evolve, driven by innovation and a commitment to patient safety, we can look forward to a future where therapeutic proteins fulfill their full potential to improve human health.