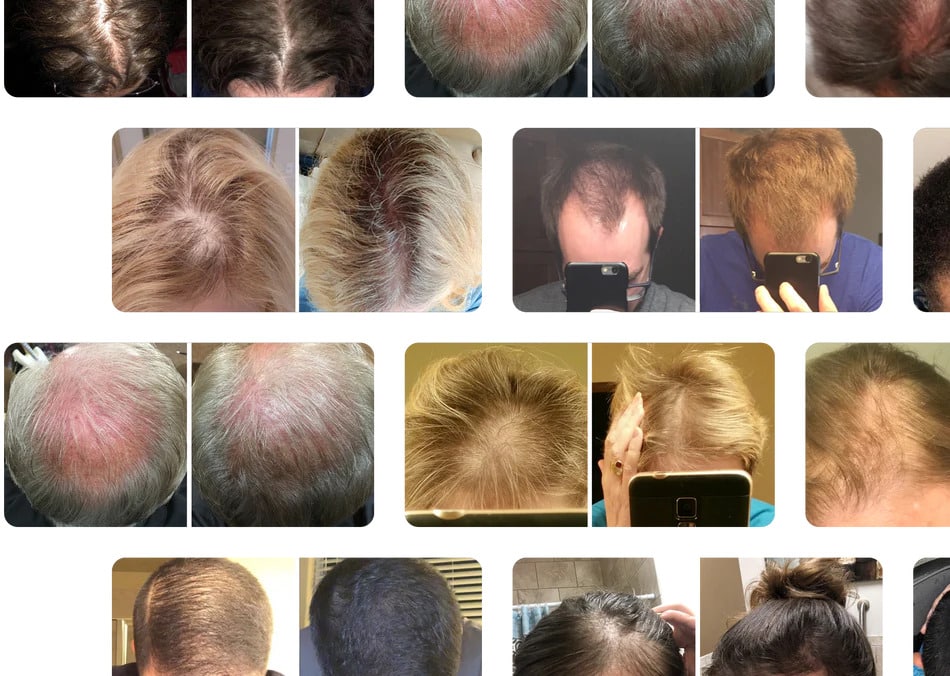
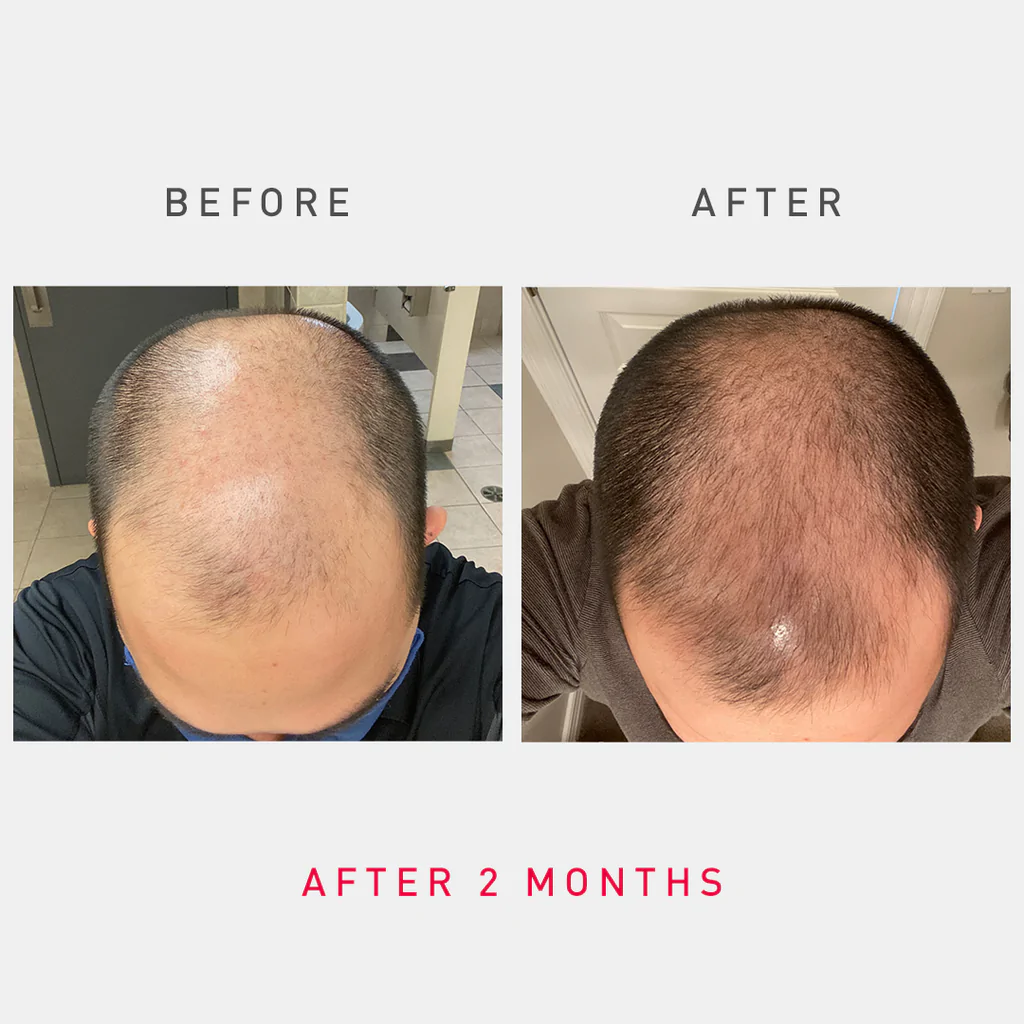
Irestore Professional 282 Laser Hair Growth Device

Hair loss, a concern affecting millions globally, is driving innovation in treatment options. From topical solutions to invasive procedures, individuals seek effective and reliable methods to combat thinning hair. The iRestore Professional 282 Laser Hair Growth Device emerges as a prominent player in this burgeoning market, promising a non-invasive approach to hair regrowth.
This article delves into the iRestore Professional 282, exploring its technology, purported benefits, clinical data, and expert opinions. It aims to provide a comprehensive overview, enabling readers to make informed decisions about this device as a potential hair loss solution. Furthermore, the piece will consider the regulatory landscape and future trends in laser-based hair restoration technology.
The Science Behind the iRestore Professional 282
The iRestore Professional 282 is a laser hair growth device that utilizes low-level light therapy (LLLT). This technology emits specific wavelengths of light, typically in the red light spectrum, which are believed to stimulate cellular activity.
Proponents suggest that LLLT enhances blood flow to the scalp, providing hair follicles with essential nutrients and oxygen. This increased stimulation is thought to revitalize dormant follicles and encourage hair regrowth.
The device incorporates 282 medical-grade lasers and LEDs, strategically positioned to maximize scalp coverage. The manufacturer, Apira Science, claims this design ensures consistent and comprehensive treatment.
How Low-Level Light Therapy Works
LLLT's mechanism of action is not fully understood, but current theories center around photobiomodulation. This process involves the absorption of light by cellular components, triggering a cascade of biochemical reactions.
These reactions are thought to include increased ATP production (cellular energy) and improved cellular metabolism. This, in turn, may lead to enhanced hair follicle activity and growth.
Skeptics, however, emphasize the need for more robust research to fully elucidate LLLT's long-term effects and efficacy.
Clinical Data and Efficacy Claims
Apira Science frequently cites clinical studies to support the efficacy of the iRestore Professional 282. These studies often report statistically significant increases in hair count among participants using the device.
One pivotal study, published in a peer-reviewed journal, showed an average increase in hair count of 43.2% after 16 weeks of treatment. However, it is crucial to critically evaluate these studies' methodology, sample size, and potential biases.
Independent researchers have called for larger, more rigorous, and blinded studies to corroborate these findings. The lack of universally accepted protocols for measuring hair growth also complicates the assessment of different treatment modalities.
User Experiences and Reviews
User reviews of the iRestore Professional 282 are mixed, reflecting the variability in individual responses to treatment. Some users report significant improvements in hair thickness and density.
Others express disappointment, citing minimal or no noticeable changes after several months of use. Common concerns include the device's price point and the time commitment required for regular treatments.
It is important to consider that individual results may vary depending on factors such as the severity of hair loss, underlying medical conditions, and adherence to the recommended treatment regimen.
Safety and Regulatory Considerations
The iRestore Professional 282 is generally considered a safe device when used as directed. LLLT is a non-invasive procedure with a low risk of side effects.
However, potential side effects may include mild scalp irritation or redness. It's crucial to consult with a dermatologist or healthcare professional before starting treatment, especially if you have underlying skin conditions.
The device is cleared by the FDA as a medical device for promoting hair growth in men and women with androgenetic alopecia. This clearance signifies that the FDA has reviewed the device and determined that it is substantially equivalent to other legally marketed devices.
Comparing iRestore to Other Hair Loss Treatments
The iRestore Professional 282 represents one of many approaches to hair loss management. Other common treatments include topical medications like minoxidil, prescription drugs like finasteride, and surgical procedures like hair transplantation.
Minoxidil, an over-the-counter topical solution, works by stimulating hair follicles and prolonging the growth phase of hair. Finasteride, a prescription medication, inhibits the production of dihydrotestosterone (DHT), a hormone linked to male pattern baldness.
Hair transplantation involves surgically moving hair follicles from denser areas of the scalp to thinning or bald areas. The choice of treatment depends on the individual's condition, preferences, and potential risks and benefits.
Expert Opinions and Perspectives
Dermatologists and hair loss specialists offer varying perspectives on the iRestore Professional 282. Some clinicians are optimistic about its potential as a non-invasive adjunct to other treatments.
Others remain cautious, emphasizing the need for more long-term studies and a better understanding of LLLT's mechanisms. A critical point to consider is that LLLT might be more effective for some types of hair loss than others.
Dr. Anya Sharma, a leading dermatologist, notes, "While LLLT shows promise, it's not a one-size-fits-all solution. A comprehensive evaluation is essential to determine the underlying cause of hair loss and tailor the treatment accordingly."
The Future of Laser Hair Growth Technology
The field of laser hair growth technology is constantly evolving. Researchers are exploring new wavelengths, energy levels, and treatment protocols to optimize efficacy and minimize side effects.
Advancements in personalized medicine may lead to more targeted LLLT treatments based on individual genetic profiles. Integrating LLLT with other hair restoration techniques, such as stem cell therapy, is also being investigated.
As technology advances and clinical evidence accumulates, laser-based hair growth devices may play an increasingly prominent role in the management of hair loss.
In conclusion, the iRestore Professional 282 represents a growing trend in non-invasive hair loss treatments. While clinical data and user experiences offer promising insights, a balanced and informed approach is crucial.
Prospective users should consult with healthcare professionals, carefully consider their individual needs and expectations, and remain critical of marketing claims. The future of hair restoration may well involve increasingly sophisticated laser technologies, but responsible adoption and rigorous evaluation will be essential.