Is A Double Bond Stronger Than A Single Bond

The question of whether a double bond surpasses a single bond in strength isn't merely an academic exercise; it's fundamental to understanding the behavior of molecules, the design of new materials, and the very processes that sustain life. The strength of chemical bonds dictates molecular stability, reactivity, and the energy released or absorbed during chemical reactions. A misunderstanding could lead to flawed predictions in fields ranging from drug development to materials science.
At its core, this article delves into the comparative strengths of single and double covalent bonds, explaining the underlying physics and chemistry. We'll dissect the nature of sigma and pi bonds, explore bond energies and lengths, and analyze specific examples to provide a comprehensive answer. We'll also address common misconceptions and consider the implications of bond strength in various chemical contexts.
Defining Bond Strength: Energy and Length
Bond strength is primarily measured by bond dissociation energy (BDE), the energy required to break a bond homolytically, meaning each atom gets one electron from the broken bond. A higher BDE indicates a stronger bond, requiring more energy to break. Bond length, the distance between the nuclei of two bonded atoms, is also an indicator: shorter bond lengths generally correlate with stronger bonds.
Typically, BDEs are expressed in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). These values are determined experimentally through techniques like calorimetry or spectroscopy. Databases of BDEs and bond lengths are crucial references for chemists and material scientists.
Sigma and Pi Bonds: The Building Blocks
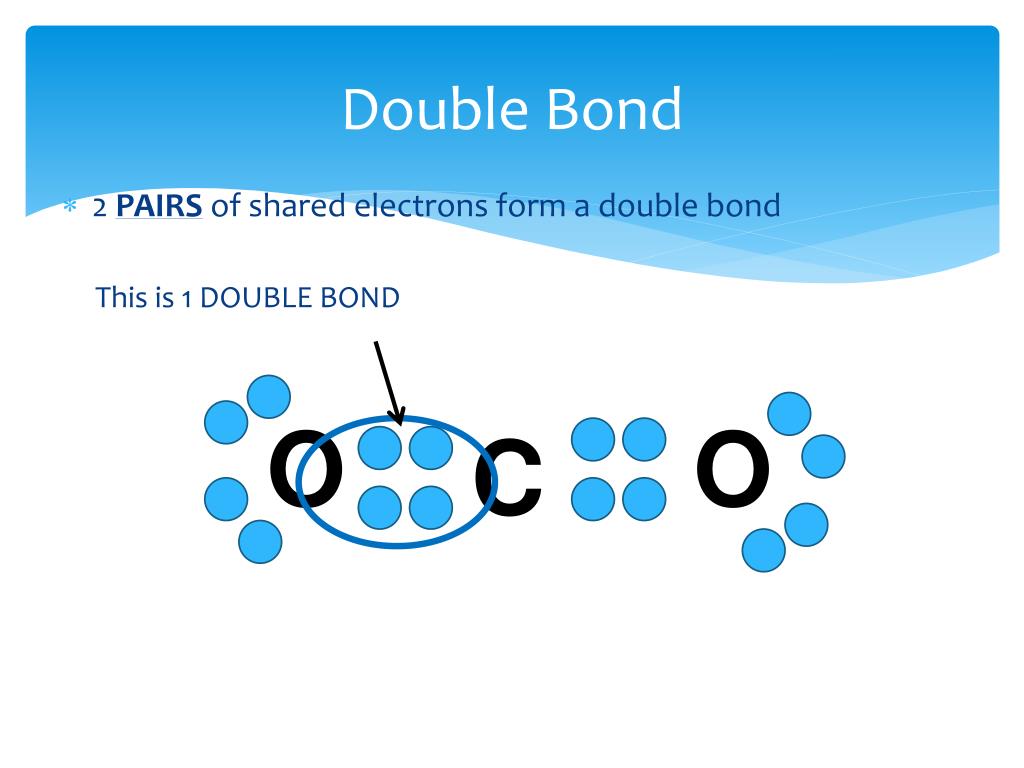
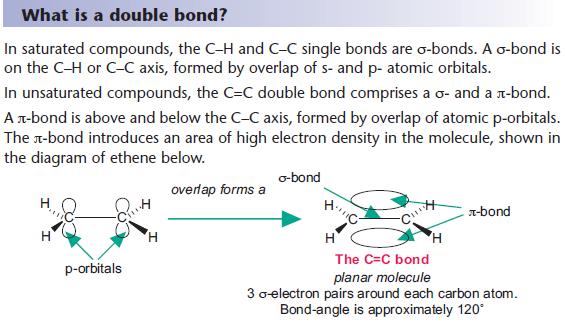
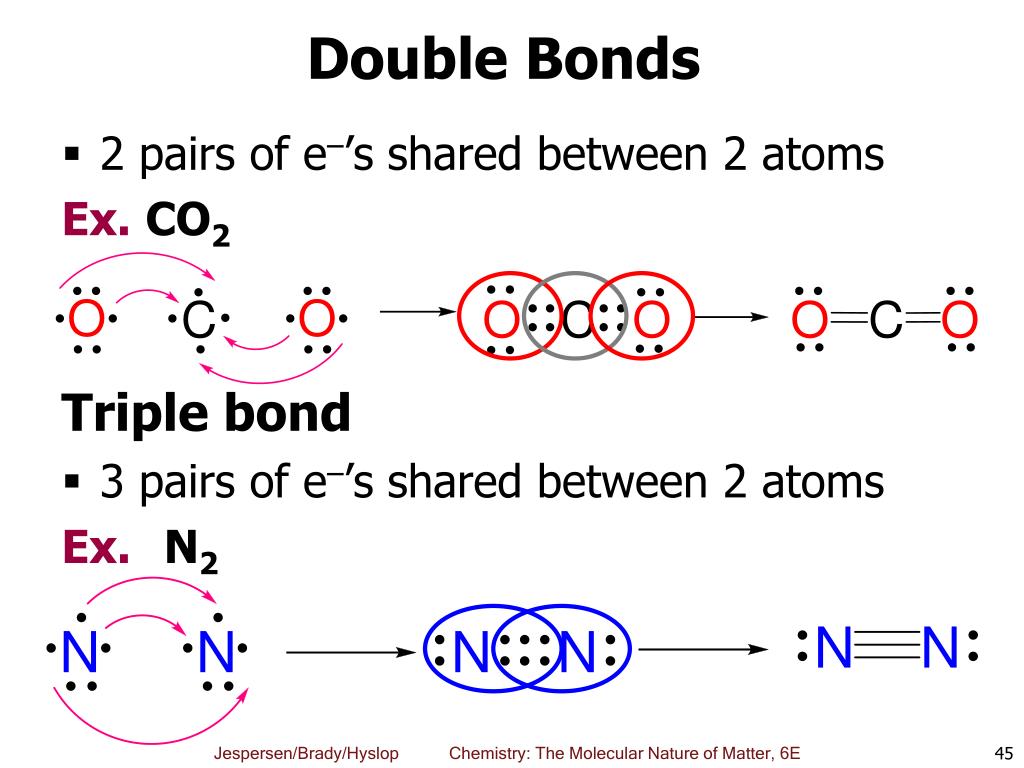
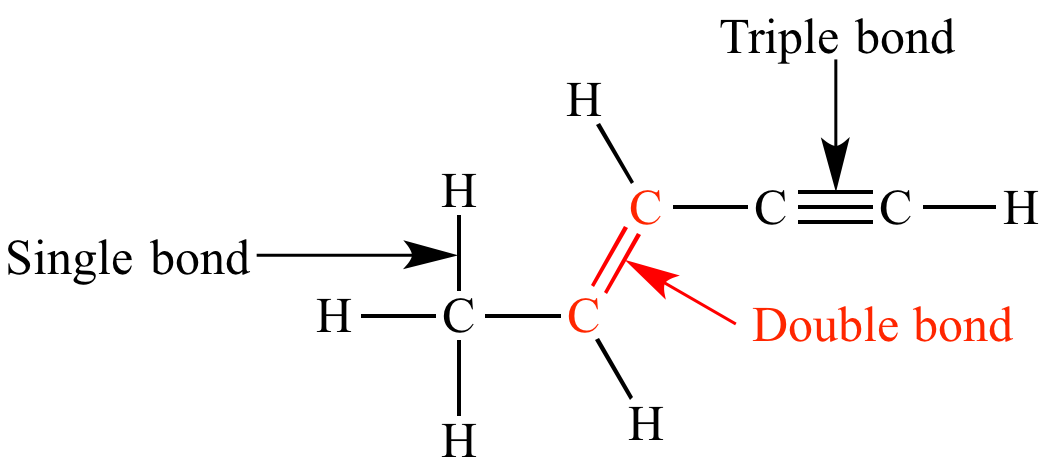
A single covalent bond consists of one sigma (σ) bond, formed by the direct, head-on overlap of atomic orbitals. This overlap creates a region of high electron density directly between the two nuclei, leading to a strong attraction. A double bond, however, comprises one sigma bond and one pi (π) bond.
The pi bond is formed by the sideways overlap of p orbitals, resulting in electron density above and below the internuclear axis. This overlap is less effective than the head-on overlap of a sigma bond, making pi bonds inherently weaker. This difference in overlap directly affects the overall bond strength.
The Double Bond Advantage: Strength, but Not Double
A double bond is indeed stronger than a single bond, but it's not twice as strong. This is because the pi bond's contribution to the overall bond strength is less than that of the sigma bond. Think of it like adding a reinforcement to a structure; it strengthens it, but not necessarily doubles its strength.
For example, the C-C single bond in ethane (H3C-CH3) has a bond energy of approximately 347 kJ/mol. The C=C double bond in ethene (H2C=CH2) has a bond energy of around 614 kJ/mol. This demonstrates that the double bond is stronger, but not twice as strong as the single bond.
Analyzing Specific Examples
Comparing the bond lengths and energies of carbon-carbon bonds further illustrates the relationship. The average C-C single bond length is approximately 154 picometers (pm), while the average C=C double bond length is around 134 pm. The shorter bond length in the double bond is consistent with its increased strength.
Similarly, consider nitrogen. The N-N single bond energy is roughly 160 kJ/mol, while the N=N double bond energy is about 418 kJ/mol. In contrast, a triple bond, containing one sigma and two pi bonds, such as in molecular nitrogen (N≡N), has a bond energy of approximately 945 kJ/mol and a bond length of 110 pm. This highlights the progressively increasing strength and decreasing length as the number of bonds increases.
Factors Influencing Bond Strength
Several factors influence bond strength beyond the number of bonds. The electronegativity difference between the bonded atoms plays a significant role. A greater electronegativity difference results in a more polar bond, often leading to increased bond strength due to electrostatic attraction.
Also, the size of the atoms involved affects bond strength. Larger atoms tend to form weaker bonds because the electron density is more dispersed. Resonance and hyperconjugation can also significantly impact bond strength by delocalizing electrons and stabilizing the molecule.
Common Misconceptions and Nuances
A common misconception is that pi bonds are always weaker than sigma bonds in all contexts. While this is generally true for simple diatomic molecules, the reactivity of a molecule often hinges on the relative ease of breaking a pi bond. Electrophilic attack, for example, often targets the pi bond of an alkene due to its higher electron density and accessibility.
It's also important to note that bond energies are average values. The actual bond energy in a specific molecule can vary depending on the surrounding atoms and the overall molecular structure. Context is key in assessing bond strength in real-world applications.
Implications in Chemistry and Materials Science
The relative strengths of single and double bonds have profound implications. In organic chemistry, the selective hydrogenation of alkynes to alkenes relies on the controlled breaking of pi bonds. This process is crucial in the synthesis of various fine chemicals and pharmaceuticals.
In materials science, understanding bond strengths is essential for designing polymers with specific properties. The strength and flexibility of a polymer chain are directly related to the types of bonds present and their resistance to breaking under stress. Strong bonds lead to durable materials, while weaker bonds allow for more flexibility.
The Future of Bond Strength Research
Research continues to push the boundaries of our understanding of chemical bonding. Advanced computational methods are allowing scientists to predict bond energies and molecular properties with increasing accuracy. The development of new catalysts and reaction conditions is also enabling the selective breaking and formation of specific bonds.
Future advancements will likely focus on understanding the interplay between bond strength, molecular structure, and environmental factors. This knowledge will be critical for developing new materials with tailored properties and designing more efficient and sustainable chemical processes. Improved understanding of chemical bonds will lead to more environmentally friendly and efficient chemical processes.
In conclusion, while a double bond is indeed stronger than a single bond, the relationship is not a simple doubling. The intricacies of sigma and pi bond interactions, coupled with other influencing factors, provide a complex and fascinating area of study that underpins much of our understanding of the molecular world. As research continues, we can expect even more nuanced insights into the nature of chemical bonding and its impact on the world around us.