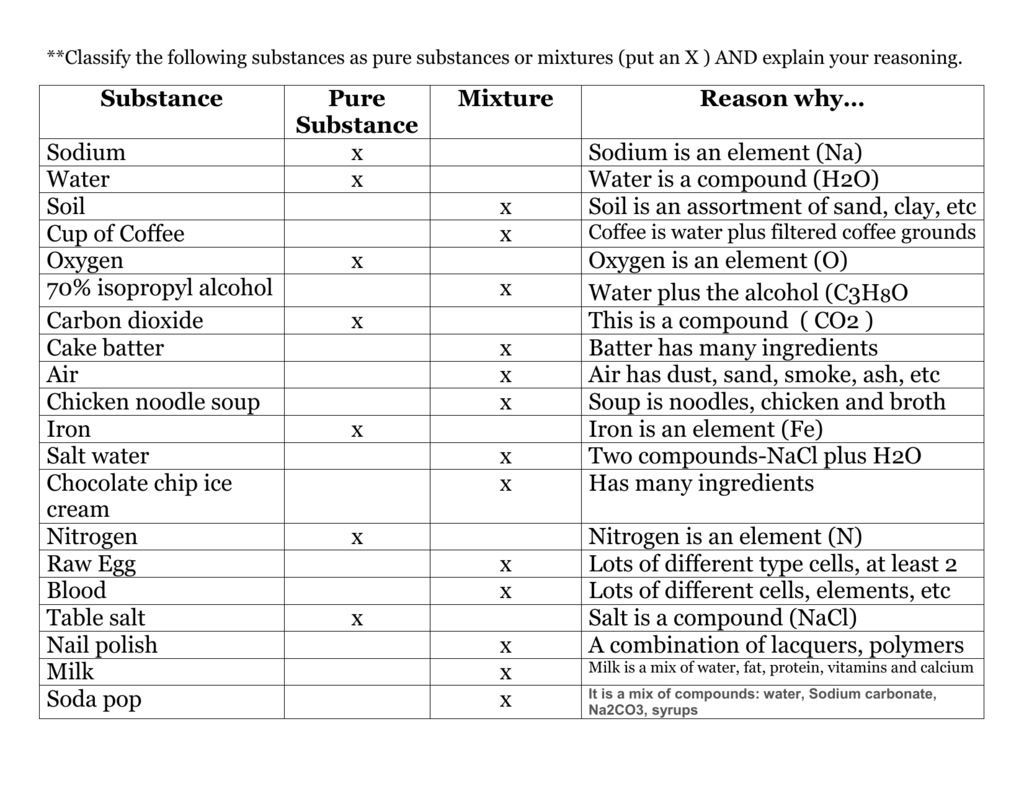
Is Salt A Mixture Or A Compound

The seemingly simple question of whether salt is a mixture or a compound has sparked debate and confusion in classrooms and online forums alike. At its core, the confusion lies in understanding the fundamental building blocks of matter and how they interact.
Is the salt we sprinkle on our food a blend of different substances, or a substance formed through the chemical combination of elements? The answer, firmly rooted in chemistry, has significant implications for how we understand and interact with the world around us.
The Nut Graf: Unpacking the Chemical Nature of Salt
This article delves into the chemical composition of salt, specifically sodium chloride (NaCl), to definitively answer whether it is a mixture or a compound. We'll explore the fundamental differences between mixtures and compounds, examining the chemical bonds that hold salt together and the scientific evidence supporting its classification as a compound.
We will also address common misconceptions and clarify why salt's consistent chemical formula disqualifies it as a mixture. The discussion will be supported by established chemical principles and verified through reputable sources.
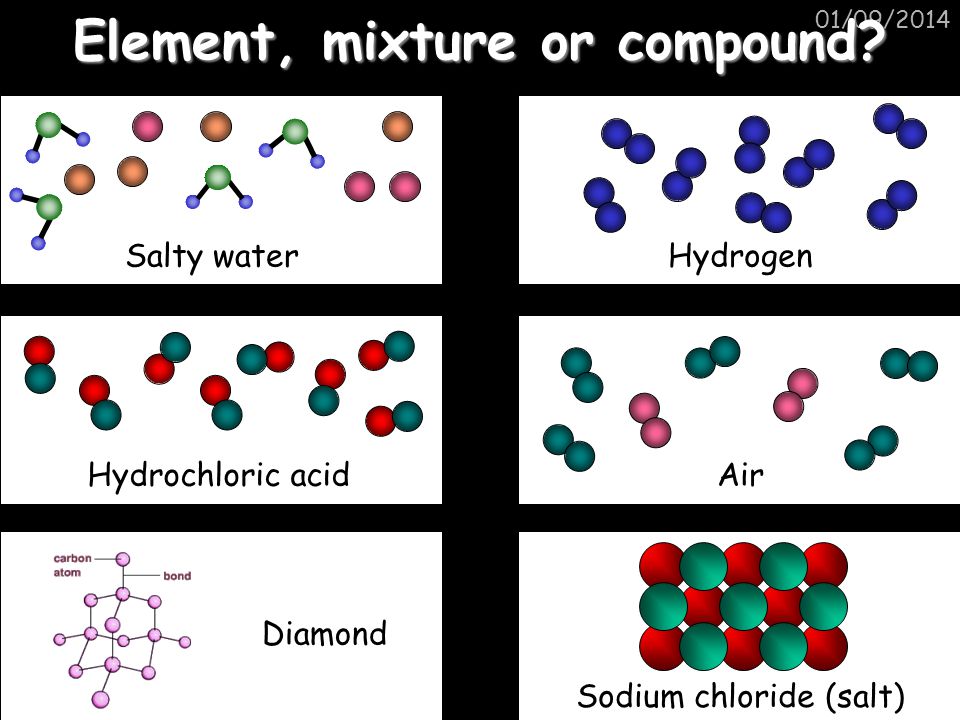
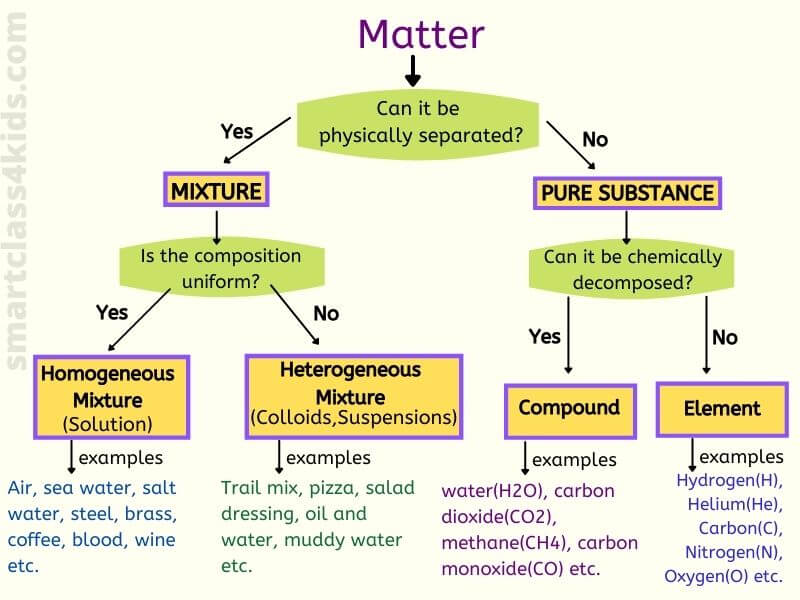
Mixtures vs. Compounds: A Fundamental Distinction
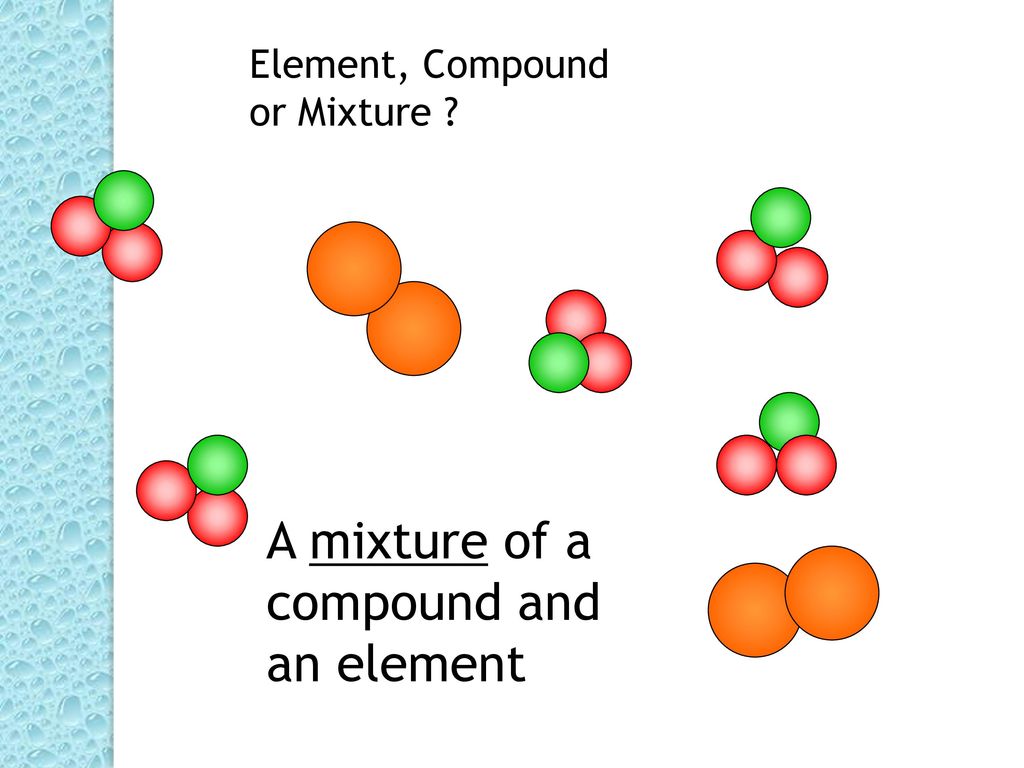
To understand the nature of salt, it’s crucial to differentiate between mixtures and compounds. Mixtures are combinations of substances that are physically combined, but not chemically bonded.
Each substance in a mixture retains its individual properties and can be separated through physical means like filtration or evaporation. Air, for example, is a mixture of nitrogen, oxygen, and other gases.
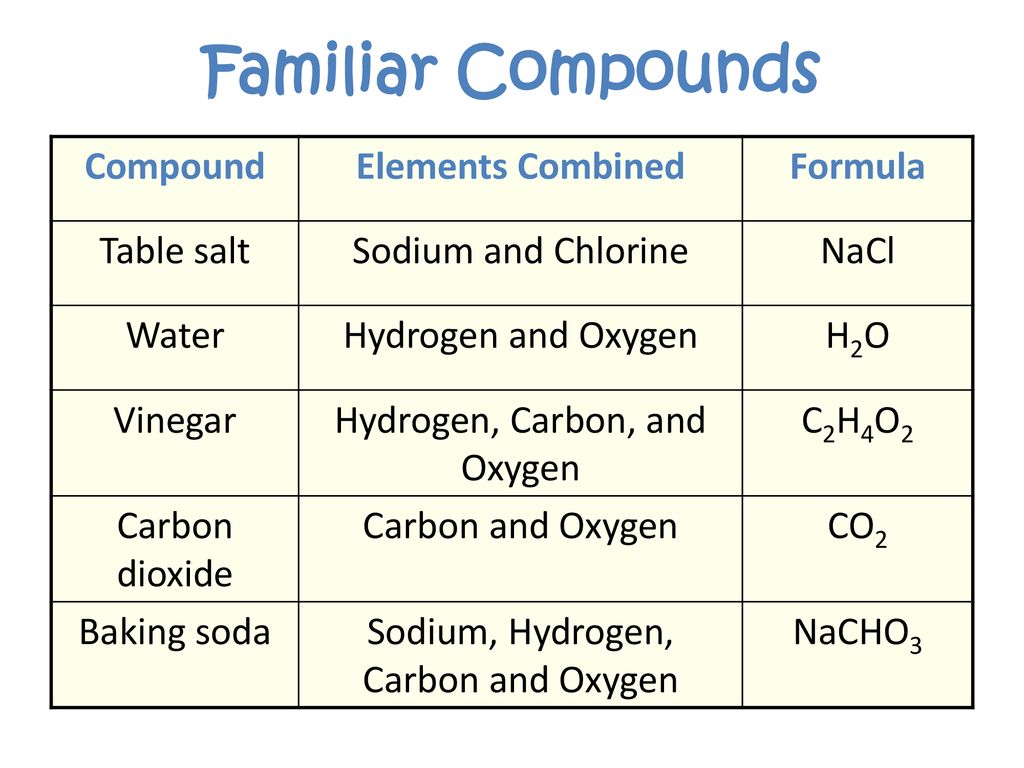
In contrast, compounds are formed when two or more elements chemically combine in a fixed ratio through chemical bonds. This chemical reaction results in a new substance with properties distinct from its constituent elements.
Sodium Chloride: A Chemical Bond Defining a Compound
Sodium chloride (NaCl), commonly known as table salt, is formed through a chemical reaction between sodium (Na), a highly reactive metal, and chlorine (Cl), a toxic gas. This reaction results in the transfer of an electron from a sodium atom to a chlorine atom.
This electron transfer creates ions: positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-). These oppositely charged ions are then strongly attracted to each other, forming an ionic bond.
This strong ionic bond creates a crystal lattice structure, where sodium and chloride ions are arranged in a repeating three-dimensional pattern. This specific and consistent arrangement is a key characteristic of a compound.
The Fixed Ratio: A Hallmark of Compounds
One of the defining characteristics of a compound is its fixed ratio of elements. In sodium chloride, the ratio is always one sodium atom to one chlorine atom (NaCl). This fixed ratio is maintained regardless of the source or amount of the salt.
This is in stark contrast to mixtures, where the proportions of the components can vary. The consistent and fixed ratio in sodium chloride provides strong evidence for its classification as a compound.
Any deviation from this 1:1 ratio would result in a different chemical substance, not simply "salt" as we know it.
Debunking Misconceptions and Addressing Ambiguity
Some confusion arises from the fact that table salt, as sold commercially, often contains additives like iodine or anti-caking agents. However, these additives are present in small amounts and do not fundamentally alter the chemical nature of the primary component, sodium chloride.
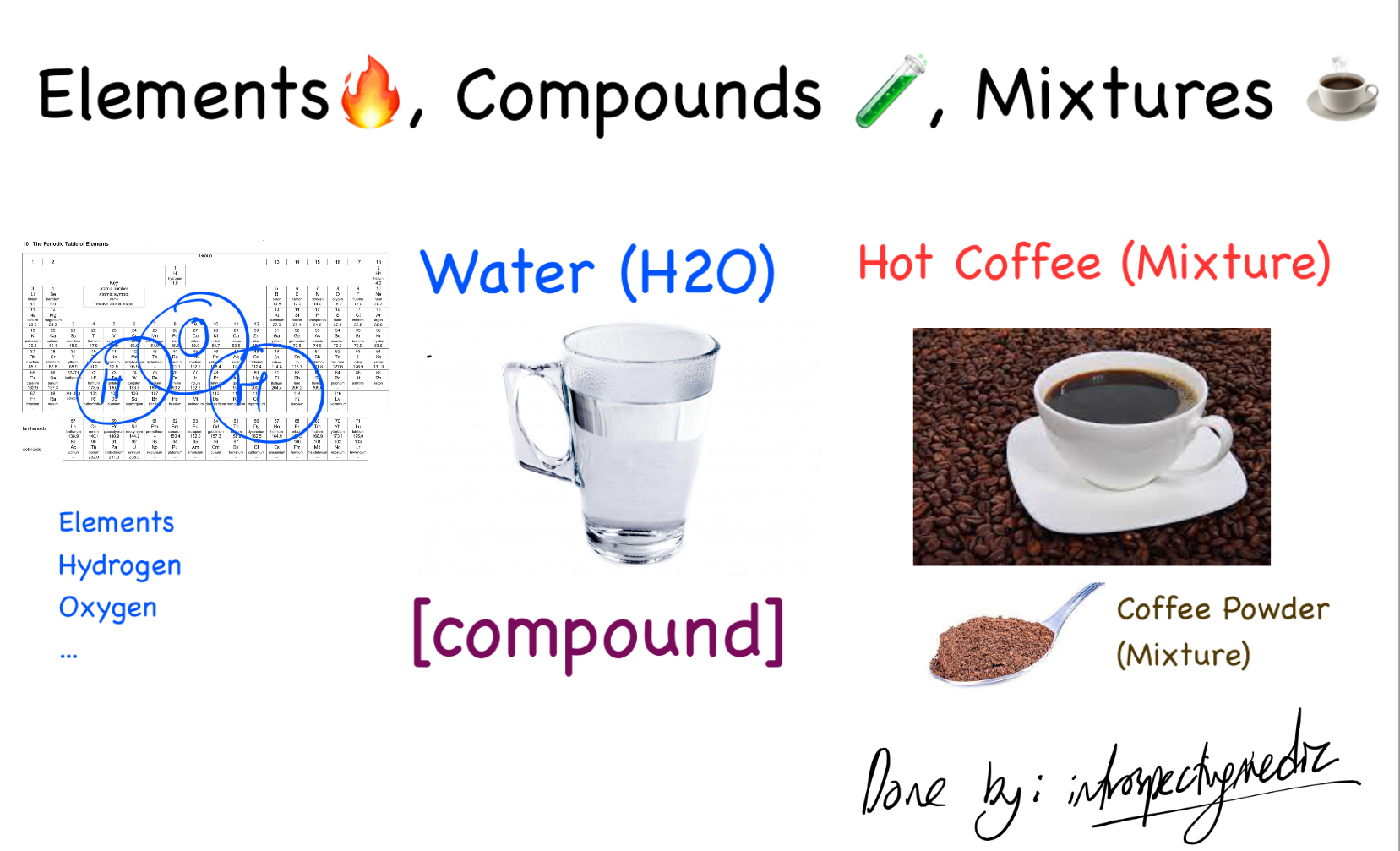
Even with these additives, the sodium chloride itself remains a compound with a defined chemical formula and properties. These additions are analogous to adding flavorings to water; the water remains H2O, even if it's now flavored.
Another point of contention sometimes raised is that salt can dissolve in water to form a solution. While this is true, dissolving a compound in a solvent does not transform the compound into a mixture. The individual sodium and chloride ions are still present, and the water is merely the medium in which they are dispersed.
Expert Opinions and Scientific Consensus
Numerous chemistry textbooks, scientific publications, and educational resources unequivocally classify sodium chloride as a compound. Chemical societies and organizations worldwide adhere to this classification.
"A compound is a substance made up of two or more different elements chemically bonded together in a fixed ratio,"
The Royal Society of Chemistry, a leading organization in the field, states, confirming that sodium chloride fits this definition perfectly.
Furthermore, research labs and universities consistently use the term "compound" when referring to sodium chloride in their experiments and publications.
Looking Ahead: Implications and Further Exploration
Understanding the distinction between mixtures and compounds is fundamental to grasping chemical principles. This knowledge is crucial for various applications, including pharmaceutical development, materials science, and environmental chemistry.
By correctly identifying sodium chloride as a compound, we gain a deeper appreciation for the chemical reactions and bonding that shape the world around us.
Further exploration of chemical bonding, crystal structures, and stoichiometry can provide a more comprehensive understanding of the properties and behavior of compounds like sodium chloride. This foundational knowledge can inspire future innovations and discoveries in the field of chemistry.

+is+a+compound.+Water+(H20)+is+a+compound..jpg)








(120).jpg)