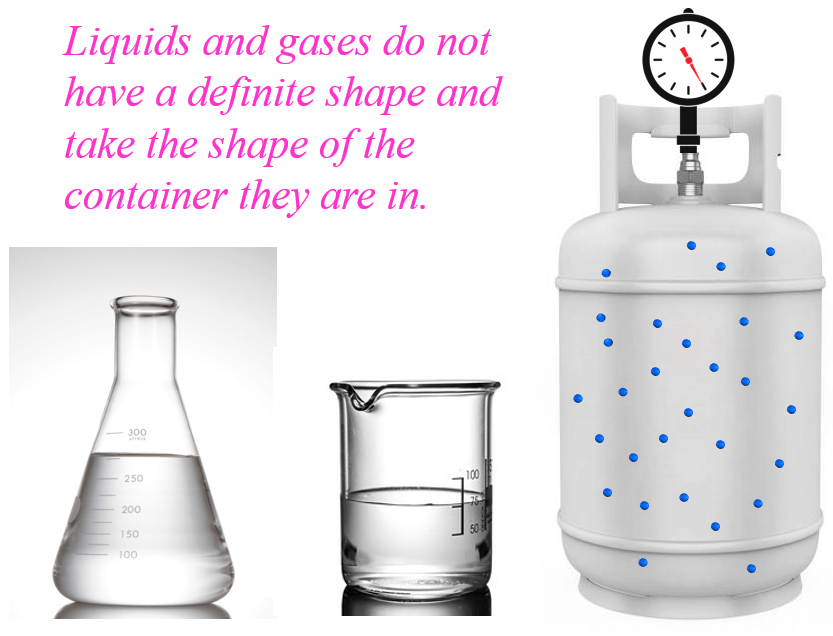Is Steam A Liquid Or A Gas

Emergency physics advisory issued: Experts are scrambling to clarify the state of steam after widespread confusion threatens scientific understanding.
The classification of steam, commonly perceived as a visible mist, is not straightforward: it's usually a gas, but the condensation we see contains liquid water droplets.
The Core Issue: Defining Steam
Steam, in its purest form, is gaseous water (H2O). This invisible gas results from water reaching its boiling point (100°C or 212°F at standard atmospheric pressure) and undergoing a phase transition.
However, what we typically perceive as "steam" is often a mixture of this gaseous water and tiny liquid water droplets formed through condensation. These droplets scatter light, making steam visible.
Understanding Phase Transitions
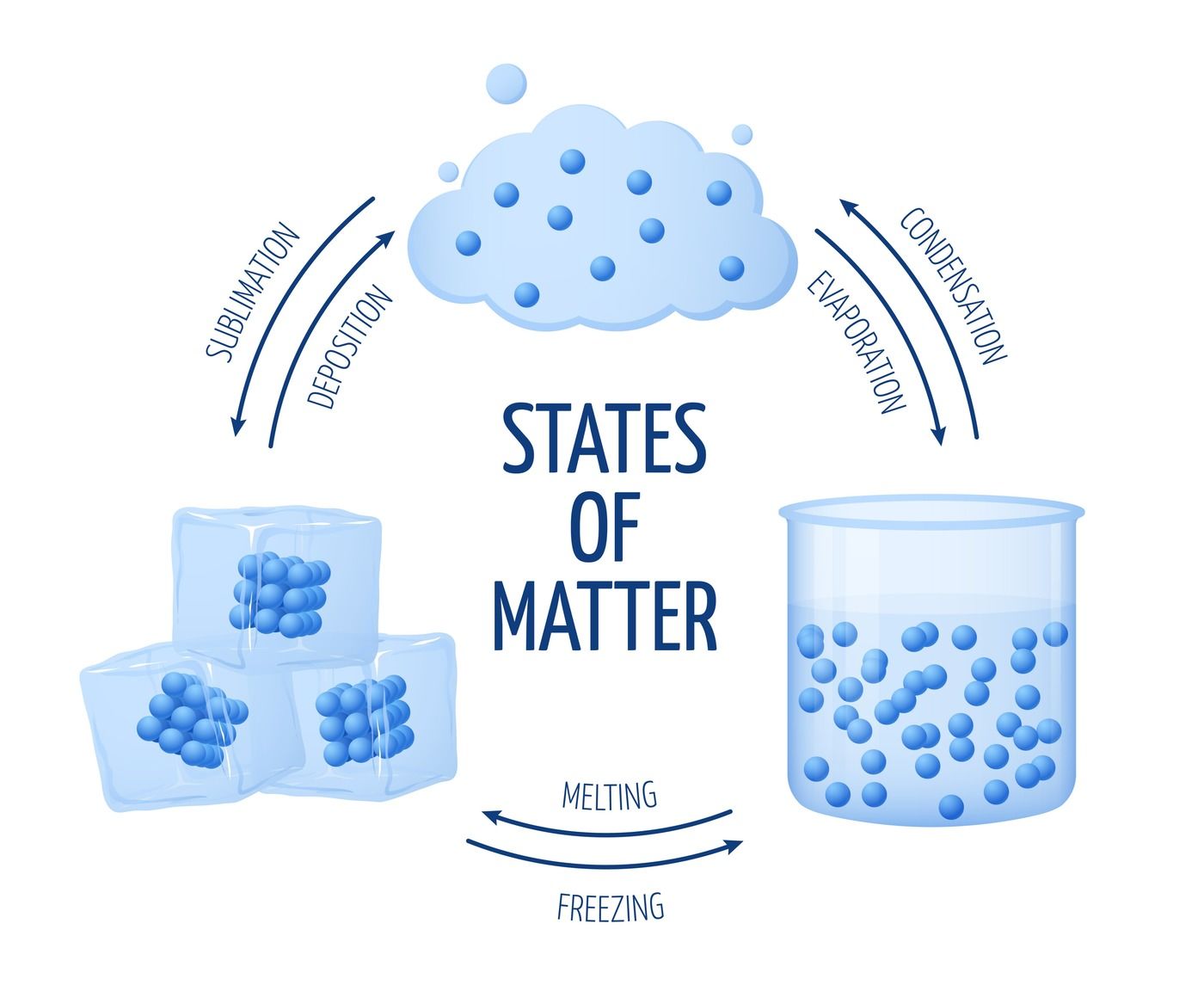
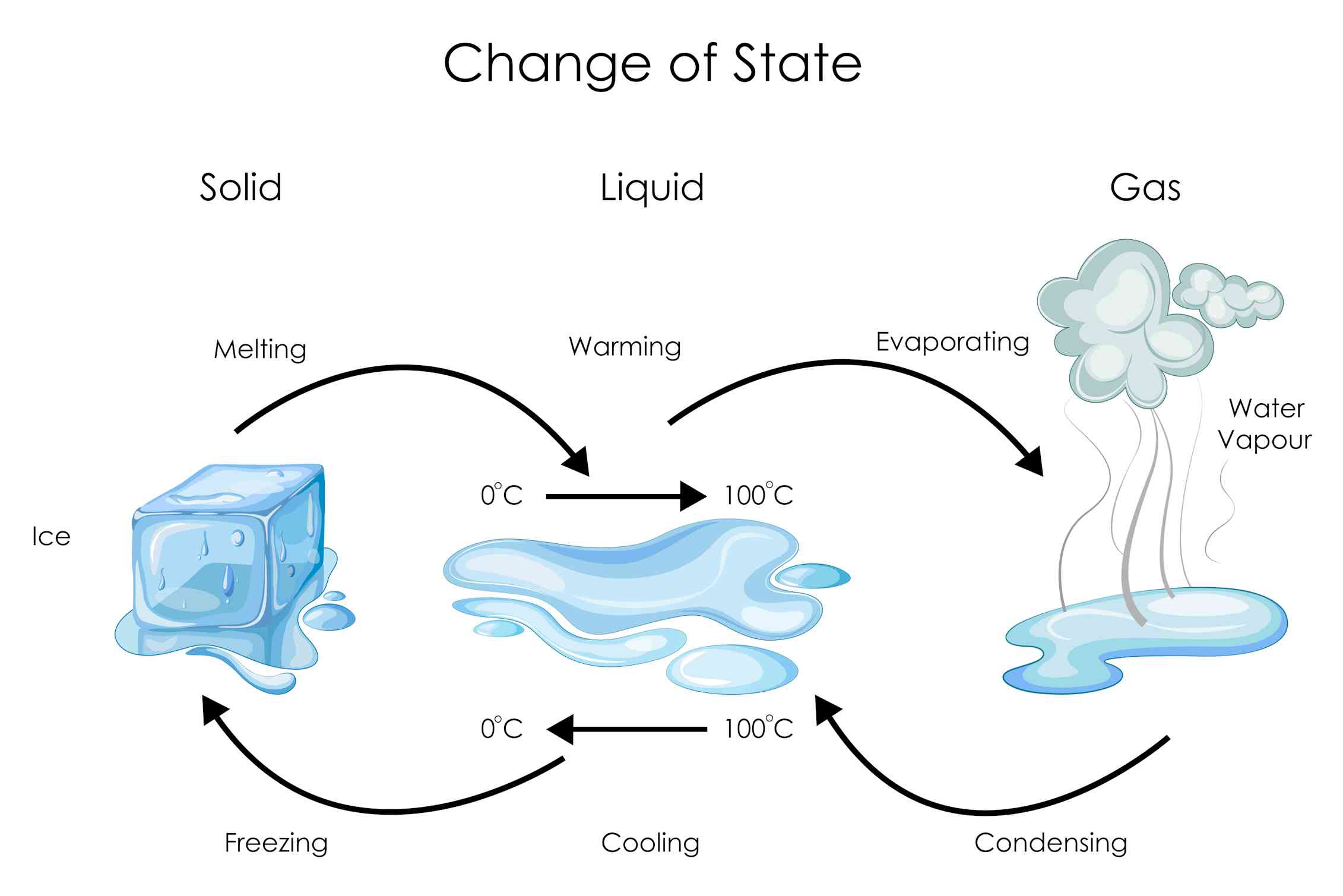
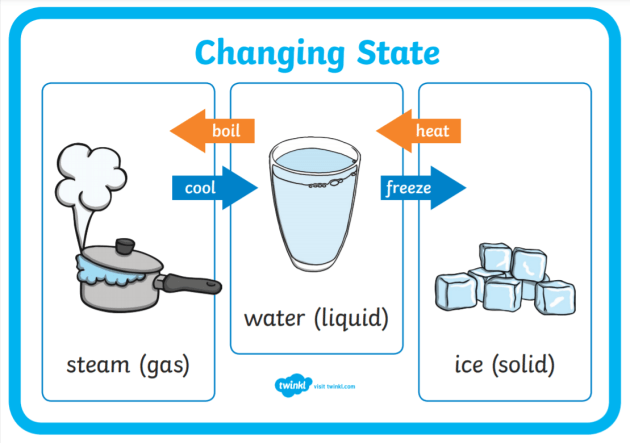
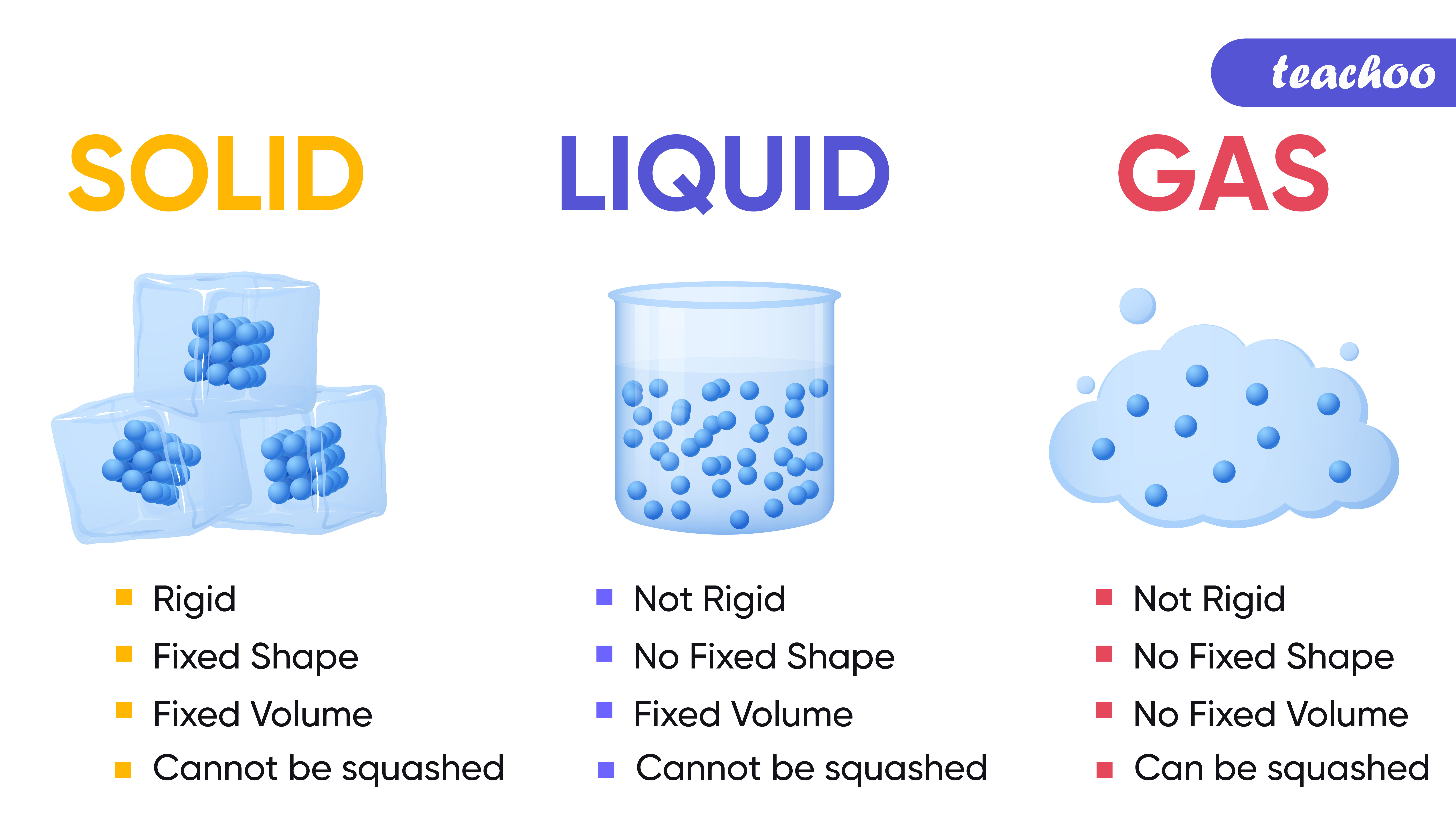
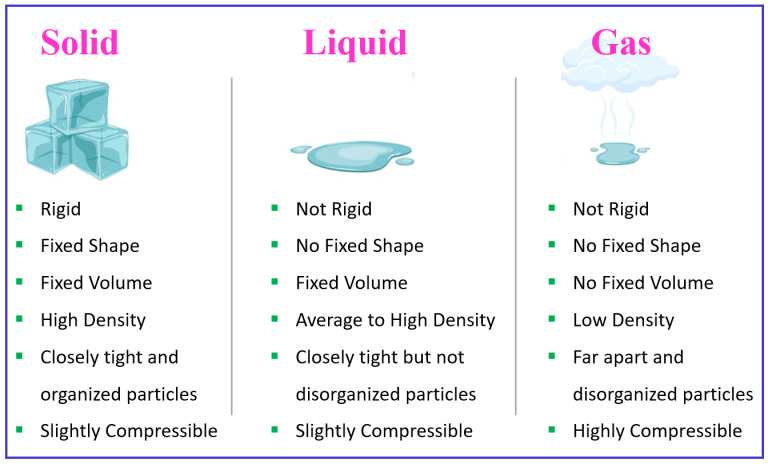
Water can exist in three phases: solid (ice), liquid (water), and gas (steam or water vapor). Transitions between these phases depend on temperature and pressure.
Boiling occurs when water reaches its boiling point and the vapor pressure equals the surrounding pressure. This causes water molecules to gain enough energy to break free from the liquid phase.
Condensation is the reverse process. As water vapor cools, its molecules lose energy, and they may clump together to form liquid droplets.
The Visible "Steam": A Closer Look
The white cloud we see rising from a kettle or a hot shower isn’t purely gaseous steam. It’s primarily condensed water droplets.
This visible mist is a consequence of the hot water vapor mixing with cooler air. The temperature difference causes the vapor to cool and condense.
The "steam" near the spout is actually closer to pure gaseous steam, hence its relative invisibility near the source.
Superheated Steam
Superheated steam is water vapor heated to a temperature significantly above its boiling point. This type of steam is entirely gaseous.
It's used in power generation and other industrial applications because it doesn't contain liquid water droplets that can damage machinery. Its high energy density makes it efficient for energy transfer.
Expert Opinions and Scientific Consensus
Dr. Emily Carter, a professor of thermodynamics at MIT, clarifies, "True steam is a colorless, odorless gas. What we see is condensed water."
According to Dr. David Lee, a chemical engineer at Stanford, "The key distinction lies in the presence or absence of liquid water. Pure steam is a gas; visible 'steam' is a mixture."
The scientific community agrees that describing visible steam as "liquid" is inaccurate, as it contains both gaseous water vapor and liquid water droplets.
Implications of Misunderstanding
The confusion surrounding steam's classification can lead to misunderstandings in various fields, from basic science education to engineering applications.
Incorrectly assuming steam is purely liquid can lead to miscalculations in energy transfer and system design. A precise understanding of its properties is crucial for efficient and safe application.
Furthermore, clear communication about the different forms of water vapor is essential for public understanding and safety around steam-generating devices.
Moving Forward: Clarification and Education
Educational institutions and science communicators are urged to emphasize the distinction between gaseous steam and condensed water vapor.
Visual aids, such as diagrams illustrating phase transitions, can help clarify the properties of steam and its different states. Public awareness campaigns are also planned.
Ongoing research focuses on improving the efficiency of steam-based technologies and minimizing the formation of liquid water droplets in industrial processes. The National Science Foundation will dedicate more fundings in this area.







+is+a+physical+change.+Solid.+ice+%3D+H2O..jpg)