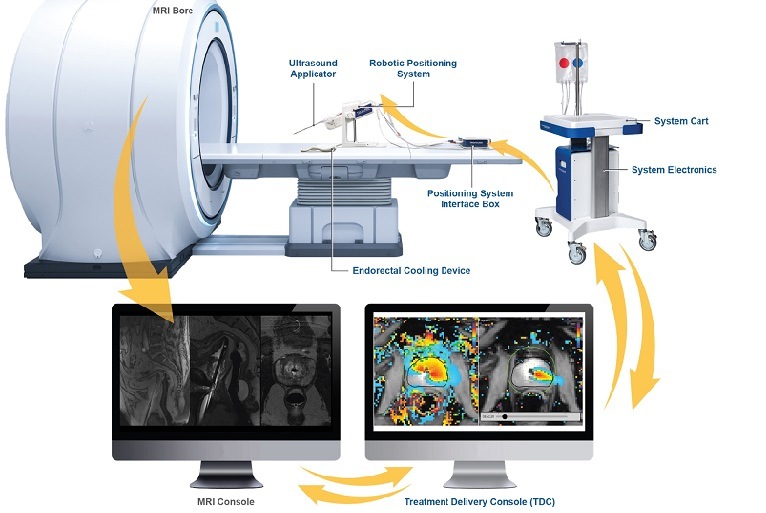
Is Tulsa Pro Covered By Medicare

The rising costs of healthcare are a constant concern for seniors and those with disabilities, particularly when it comes to specialized medical devices. A pressing question for many in the Tulsa area and beyond is whether Tulsa Pro, a device purported to address specific medical needs, is covered by Medicare. The answer isn't simple, and understanding the nuances can significantly impact a patient's financial burden and access to care.
At the heart of the matter is Medicare's complex coverage determination process. This article delves into whether Tulsa Pro falls under Medicare's covered devices, examining specific criteria, related Durable Medical Equipment (DME) policies, and expert opinions. We'll also explore alternative avenues for potential financial assistance and the broader implications for patients seeking access to this technology.
Understanding Medicare Coverage
Medicare coverage is typically determined by several factors. These factors include medical necessity, device classification, and whether the device is considered reasonable and necessary for the treatment of a specific medical condition. Many devices are reviewed under the category of Durable Medical Equipment (DME), which has specific guidelines.
DME is defined as equipment that can withstand repeated use. It must be primarily and customarily used to serve a medical purpose. And generally is not useful to a person in the absence of illness or injury. Importantly, it must be appropriate for use in the home.
For Tulsa Pro to be covered under Medicare as DME, it needs to meet all of these criteria. Whether it actually satisfies these points is a key sticking point and is addressed further below.
Is Tulsa Pro Classified as DME?
Determining whether Tulsa Pro is classified as DME is a crucial step. Without that classification, it is impossible to proceed toward potential reimbursement. The manufacturer's specifications and intended use are essential in this determination.
Currently, information specifically classifying Tulsa Pro within established DME categories is limited. Official Medicare databases and coding systems are generally referenced when determining DME status. This lack of a clear classification creates an initial hurdle.
Medical Necessity and Reasonable & Necessary Standards
Even if Tulsa Pro were classified as DME, it still needs to meet the standard of being "medically necessary." This means the device must be reasonable and necessary for the diagnosis or treatment of an illness or injury. It also must improve or maintain the patient's functional capacity.
Medicare requires substantial clinical evidence to support the medical necessity of any device. Studies and physician testimonies highlighting the device's effectiveness in treating specific medical conditions are crucial for proving medical necessity. This evidence should be peer-reviewed and show the Tulsa Pro offers a benefit over existing treatments.
The "reasonable and necessary" standard often requires a device to be proven effective in treating a specific condition. It also necessitates that the device not be experimental or investigational. Clear treatment protocols and guidelines for using the device are generally required.
The Role of Local Coverage Determinations (LCDs)
Medicare coverage decisions can vary based on geographic location through Local Coverage Determinations (LCDs). LCDs are decisions made by Medicare Administrative Contractors (MACs) regarding whether to cover a particular item or service in their specific jurisdiction.
These LCDs take into account local medical practices. They also consider the specific health needs of the population within the region. Searching for LCDs related to devices similar to Tulsa Pro in Oklahoma or neighboring states can provide valuable insight.
Absence of specific LCDs for Tulsa Pro could suggest the device is not yet widely recognized or covered. It could also mean there have been no specific requests for coverage and subsequent denials that result in formal LCD development.
Seeking Alternative Financial Assistance
If Medicare does not cover Tulsa Pro, patients may explore alternative avenues for financial assistance. These could include private insurance, manufacturer assistance programs, and charitable organizations focused on providing medical equipment.
Many private insurance plans offer more comprehensive coverage than Medicare. Reviewing one’s private insurance policy to determine coverage for durable medical equipment and similar devices is crucial. Sometimes pre-authorization from a doctor is required.
Certain manufacturers offer patient assistance programs to help offset the cost of their devices. Additionally, organizations like the American Cancer Society or local charities might provide funding for medical equipment for patients who meet certain criteria.
The Broader Implications and Future Outlook
The issue of Medicare coverage for devices like Tulsa Pro highlights the ongoing challenges of healthcare access. It underscores the need for clear and transparent coverage policies regarding innovative medical technology.
As medical technology continues to advance, the healthcare system must adapt by establishing robust evaluation processes. These evaluations should be done to determine the value, safety, and effectiveness of new devices and therapies.
Patients and healthcare providers should actively engage in advocating for fair and equitable access to innovative medical technologies. Such advocacy involves supporting clinical trials, providing feedback to policymakers, and educating the public about the benefits of these devices.

.jpg)