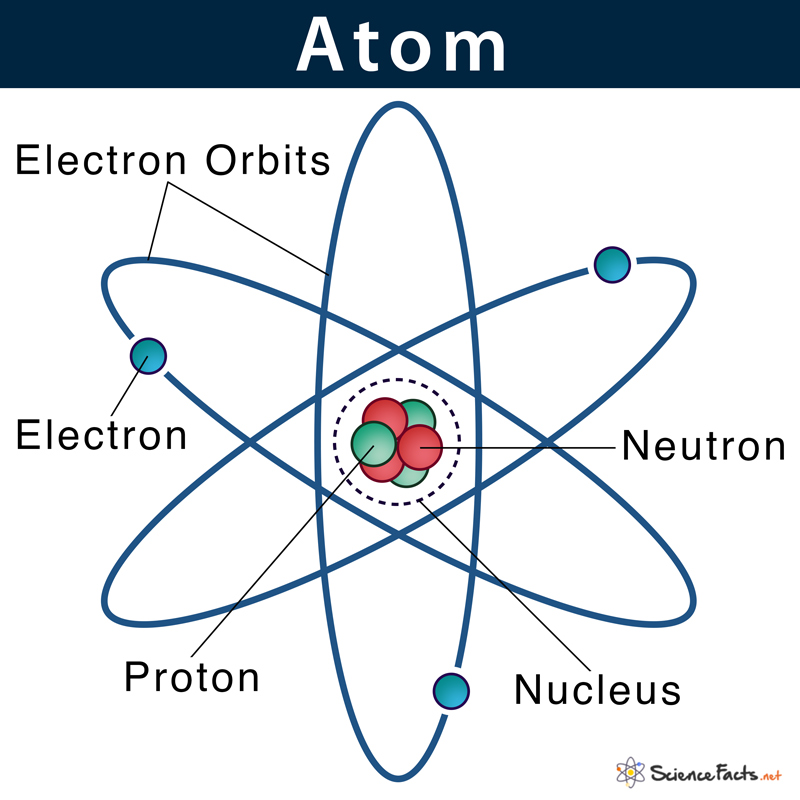
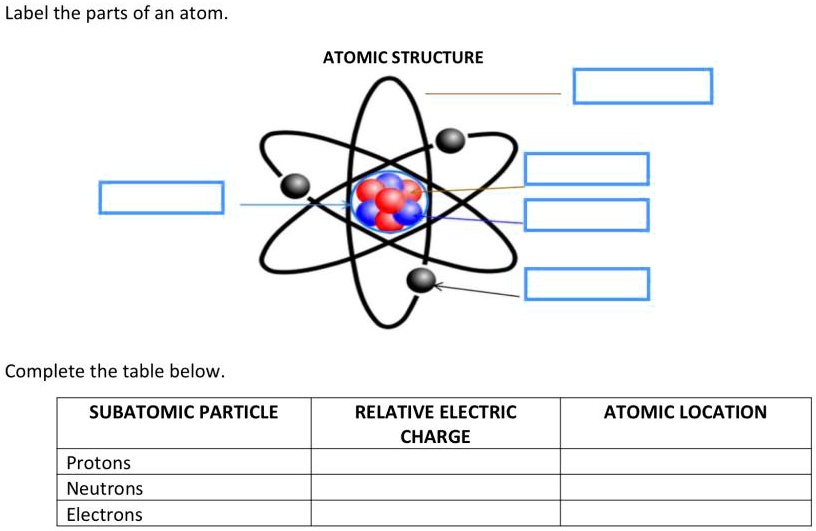
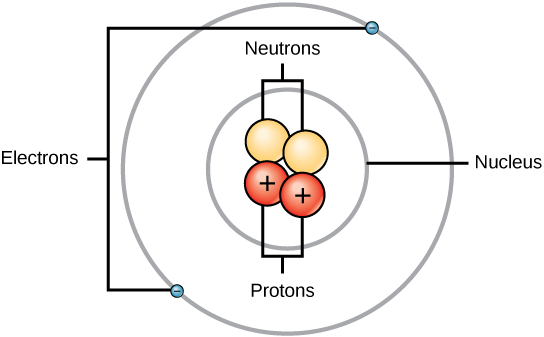
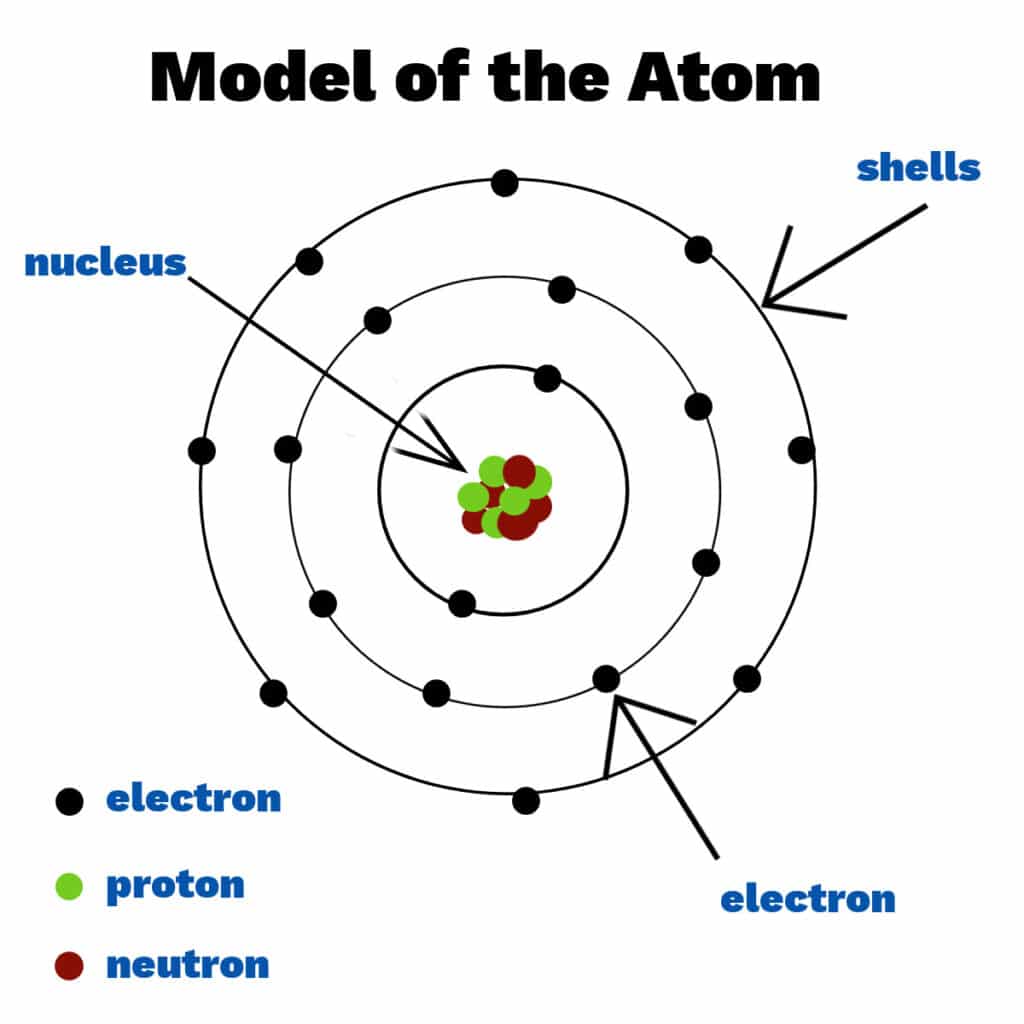
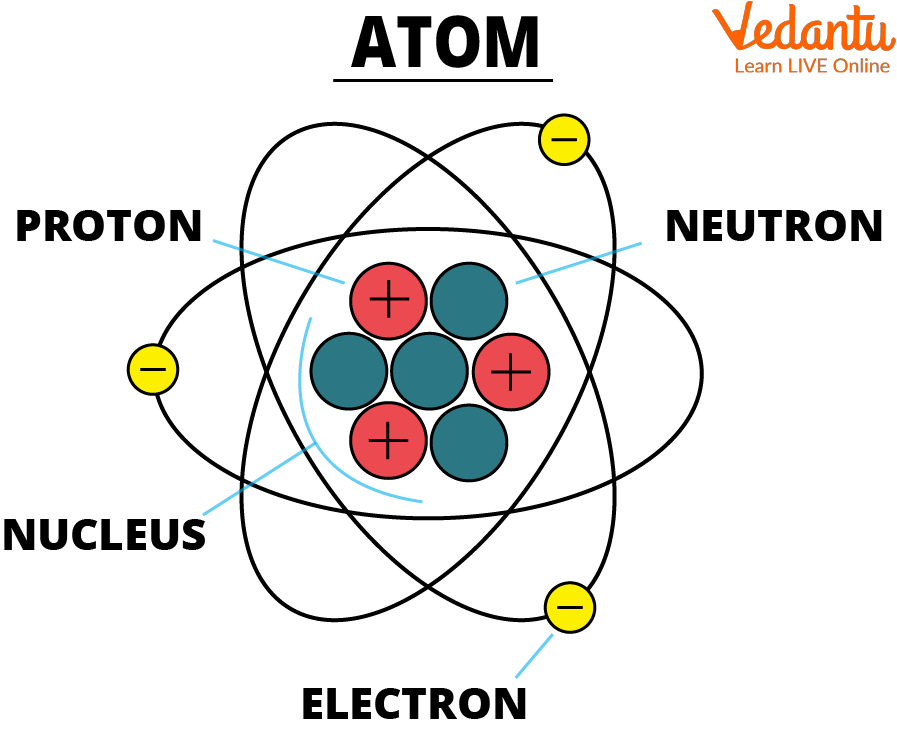
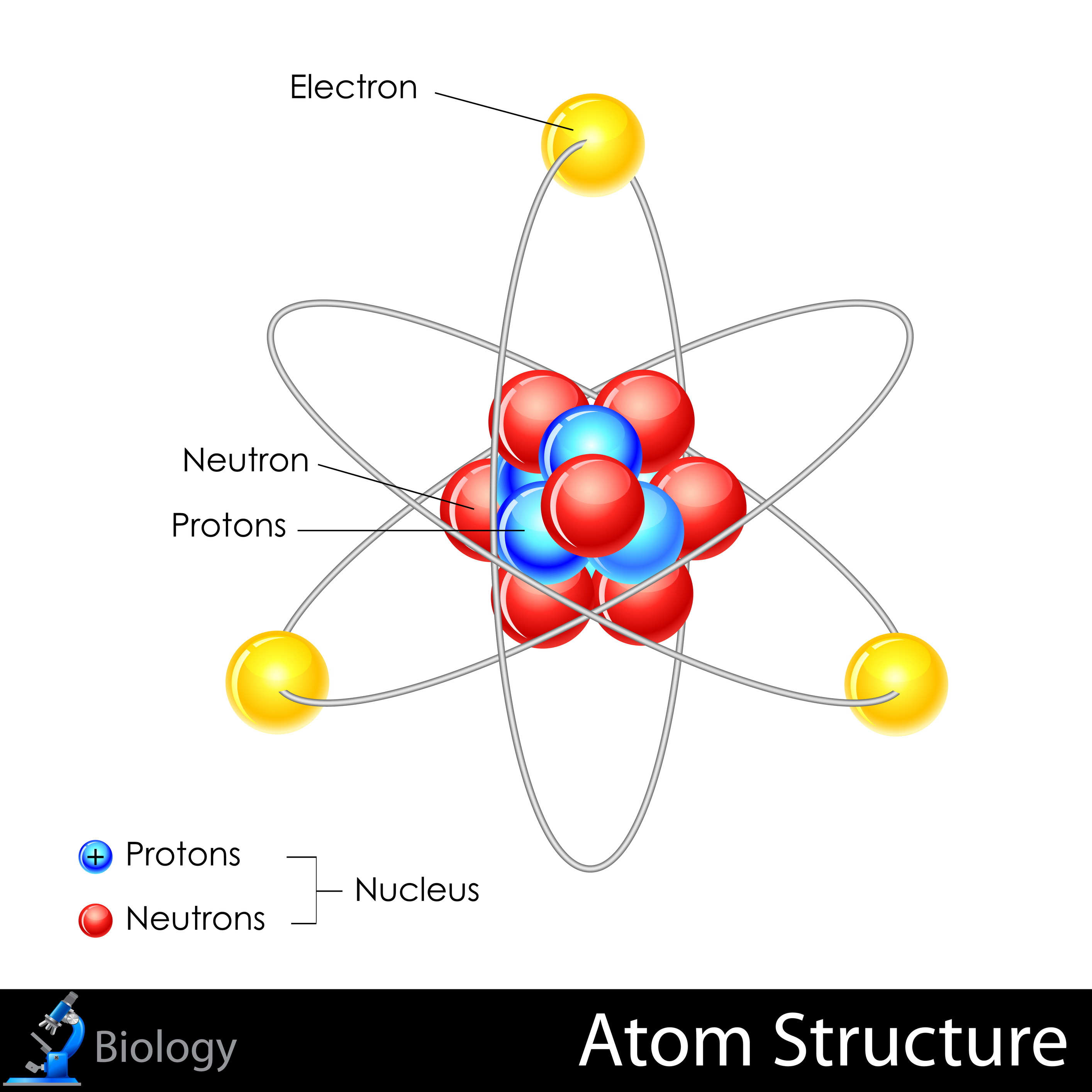
Label Each Model Of An Atom With Its Appropriate Information

For centuries, humanity has strived to understand the fundamental building blocks of matter. From philosophical musings to rigorous scientific experimentation, the concept of the atom has evolved dramatically. Accurately understanding and labeling each model of the atom is crucial for grasping the progression of scientific thought and the foundations of modern chemistry and physics.
This article explores the key atomic models that have shaped our understanding of matter, providing a clear identification of each model with its core features, proponents, and limitations. We will trace the journey from the earliest philosophical ideas to the complex quantum mechanical models that underpin our current understanding, highlighting the critical experiments and theoretical breakthroughs that propelled these paradigm shifts. By clearly delineating each model, we aim to provide a comprehensive overview of atomic theory for students, educators, and anyone interested in the history of science.
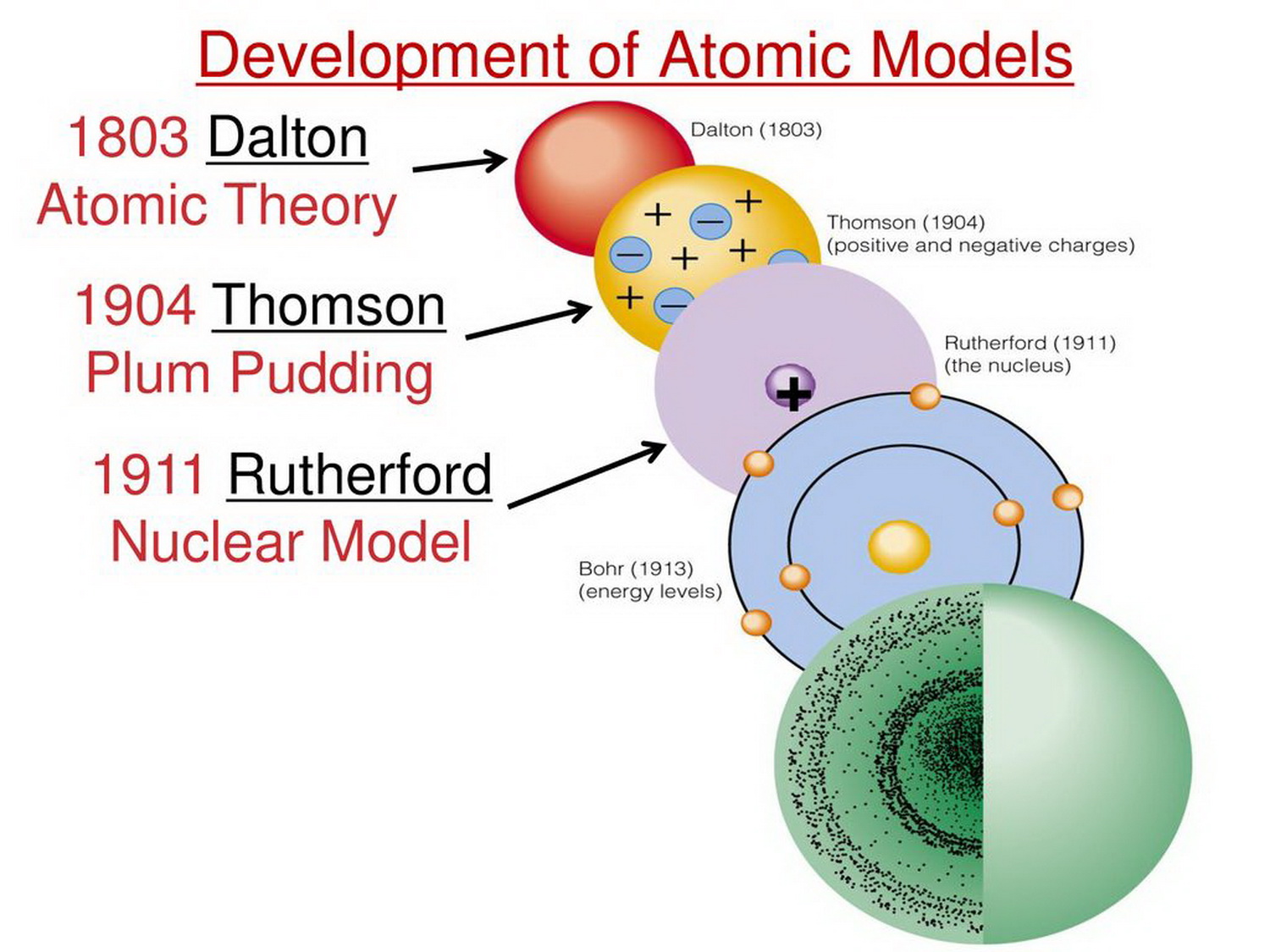
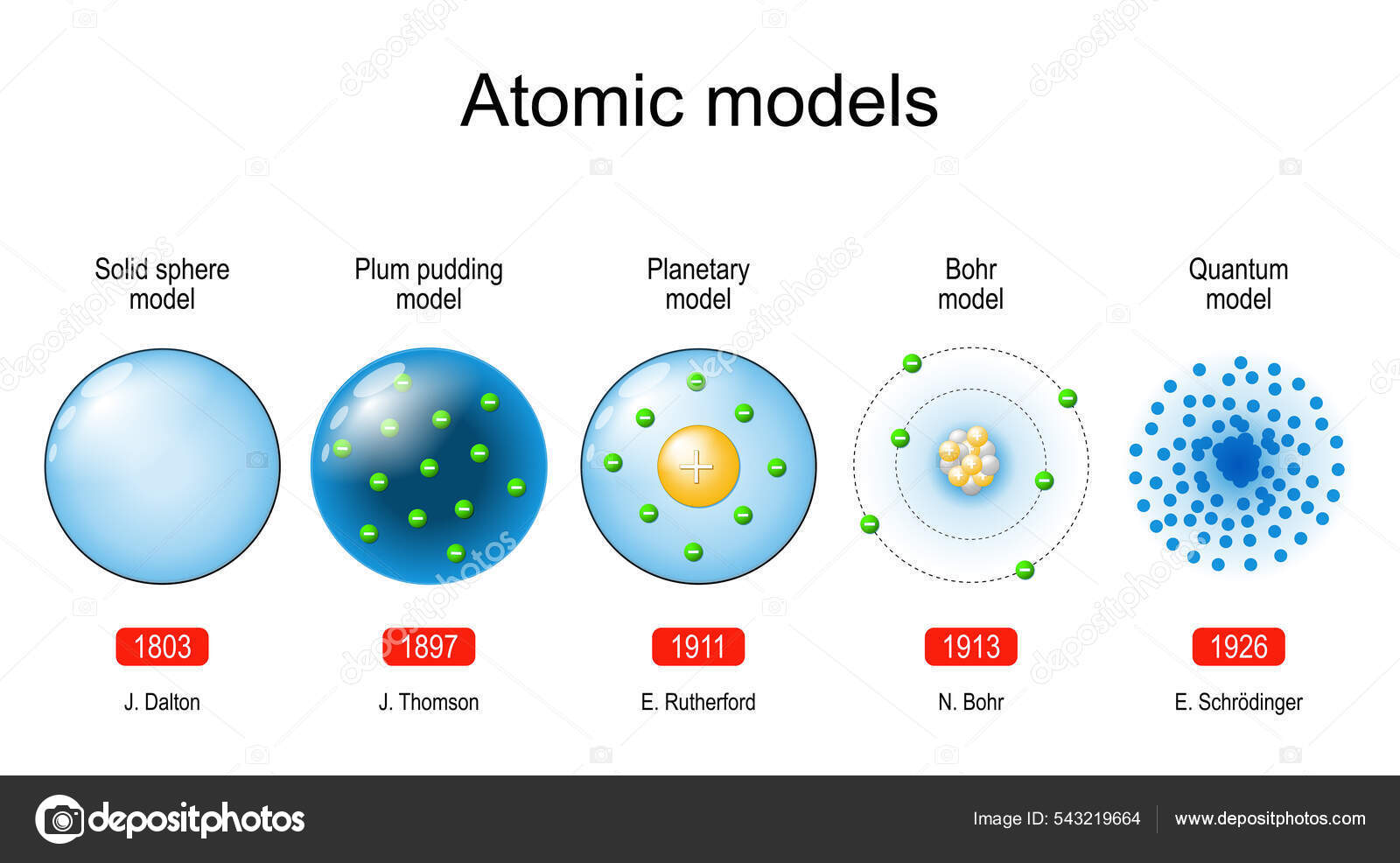
Dalton's Atomic Model (1803)
John Dalton, an English chemist and physicist, proposed the first scientific atomic theory. His model, presented in 1803, posited that all matter is composed of indivisible and indestructible atoms. He further stated that all atoms of a given element are identical in mass and properties, and compounds are formed by a combination of two or more different kinds of atoms.
Dalton's model provided a foundation for understanding chemical reactions and stoichiometry. However, it lacked any internal structure for the atom and could not explain phenomena like electrical conductivity. The simplicity of the model was both its strength and its ultimate limitation.
Thomson's Plum Pudding Model (1904)
J.J. Thomson, discoverer of the electron, proposed the "plum pudding" model in 1904. This model envisioned the atom as a sphere of positive charge with negatively charged electrons embedded within it, much like plums in a pudding. The positive and negative charges were thought to be evenly distributed, resulting in a neutral atom.
Thomson's model was a significant step forward, acknowledging the existence of subatomic particles. The model failed to explain the results of the Rutherford gold foil experiment, which demonstrated that the atom's positive charge was concentrated in a small, dense nucleus.
Rutherford's Nuclear Model (1911)
Ernest Rutherford, along with his assistants Hans Geiger and Ernest Marsden, conducted the famous gold foil experiment. They bombarded a thin gold foil with alpha particles and observed that some particles were deflected at large angles, contrary to Thomson's model. Rutherford interpreted this as evidence of a small, dense, positively charged nucleus at the center of the atom, with electrons orbiting around it.
Rutherford's model was a revolutionary departure from the plum pudding model. It correctly identified the nucleus and the orbiting electrons but failed to explain why these electrons did not spiral into the nucleus due to electromagnetic radiation, a problem classical physics couldn't solve.
Bohr's Atomic Model (1913)
Niels Bohr, building upon Rutherford's model, introduced the concept of quantized energy levels for electrons. Bohr proposed that electrons could only orbit the nucleus in specific orbits with discrete energy levels, and they could jump between these levels by absorbing or emitting energy in the form of photons. This model incorporated Planck's quantum theory.
Bohr's model successfully explained the discrete spectral lines of hydrogen. However, it failed to accurately predict the spectra of more complex atoms and was based on postulates that seemed somewhat arbitrary and ad hoc. It was a stepping stone towards a more complete quantum mechanical description.
The Quantum Mechanical Model (1920s-Present)
The quantum mechanical model, developed by Erwin Schrödinger, Werner Heisenberg, and others, provides the most accurate and complete description of the atom to date. It treats electrons as having wave-like properties and introduces the concept of atomic orbitals, which are regions of space where electrons are most likely to be found. This model relies heavily on probability and wave equations.
Unlike Bohr's model, the quantum mechanical model does not specify exact electron orbits. Instead, it describes electron distribution in terms of probabilities and quantum numbers. The model is based on the Heisenberg Uncertainty Principle, which states that it is impossible to simultaneously know both the position and momentum of an electron with perfect accuracy.
The quantum mechanical model is incredibly successful in explaining the chemical behavior of atoms and molecules. It provides the theoretical foundation for understanding chemical bonding, molecular structure, and a wide range of chemical phenomena. It relies on the Schrödinger equation to describe the behavior of electrons in atoms.
Future Directions
While the quantum mechanical model is the most sophisticated model we have, research continues to refine our understanding of the atom. Scientists are exploring new computational methods to solve the Schrödinger equation for increasingly complex systems. Additionally, research into exotic forms of matter, such as antimatter and strange matter, continues to challenge and expand our understanding of fundamental physics.
The ongoing quest to understand the atom reflects humanity's inherent desire to unravel the mysteries of the universe. By accurately labeling and understanding each atomic model, we appreciate the scientific process and stand ready for future discoveries.