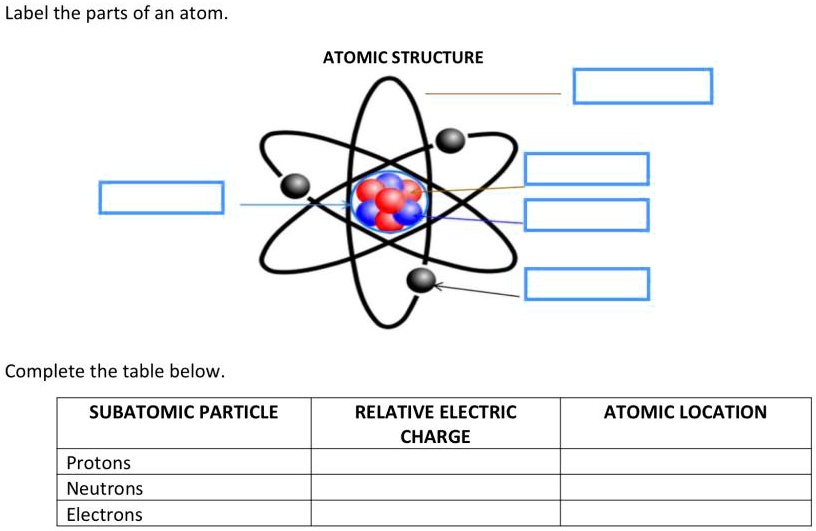
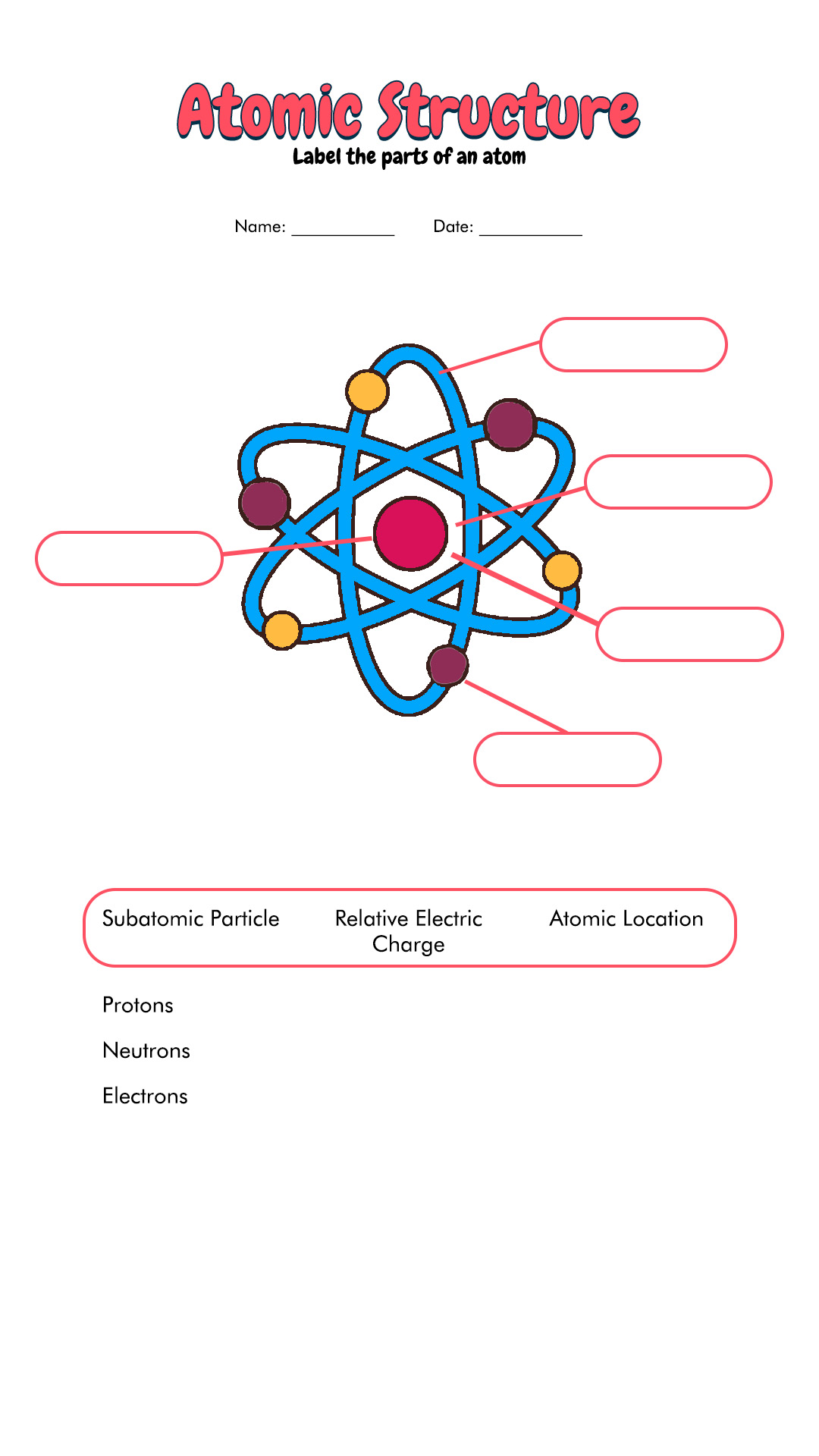
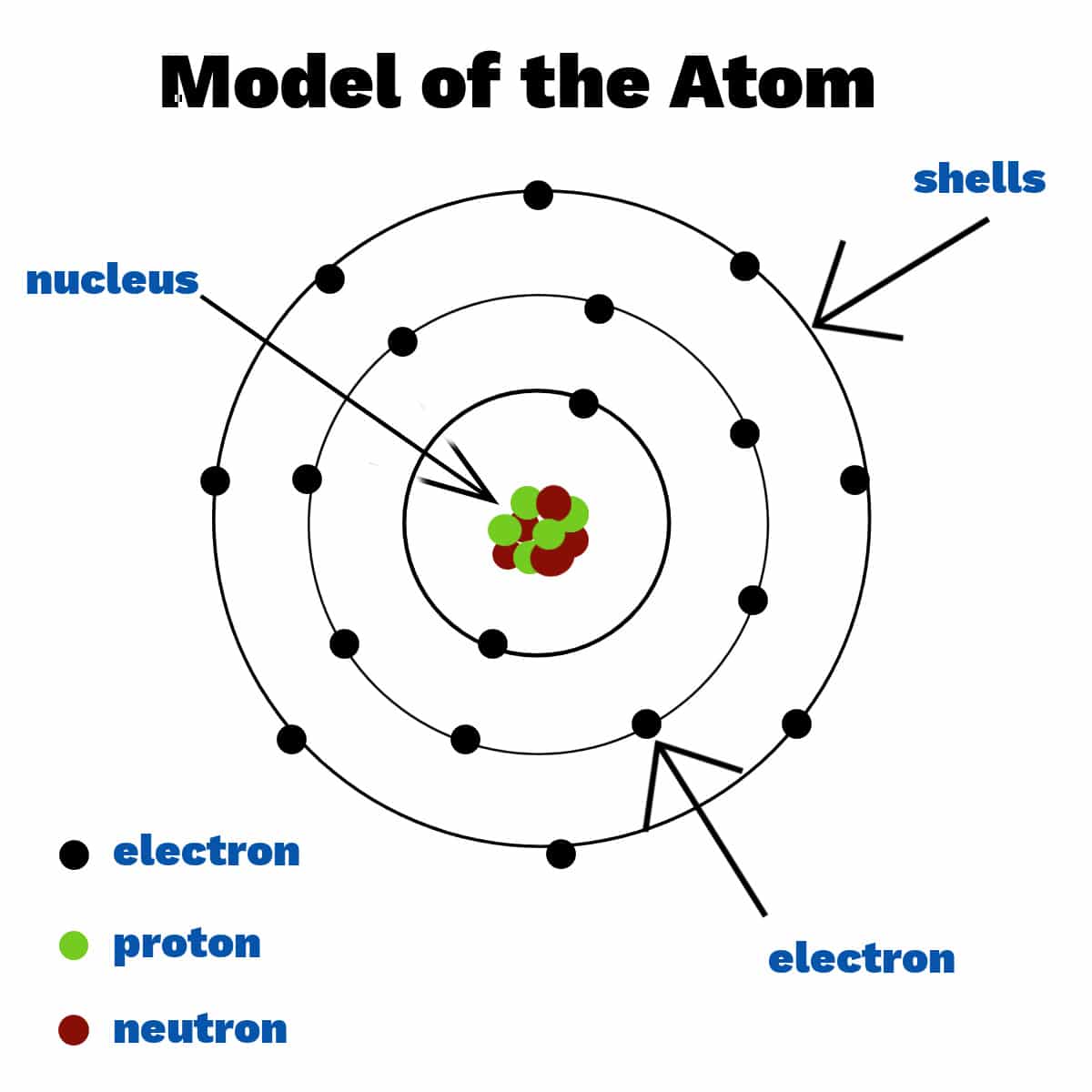
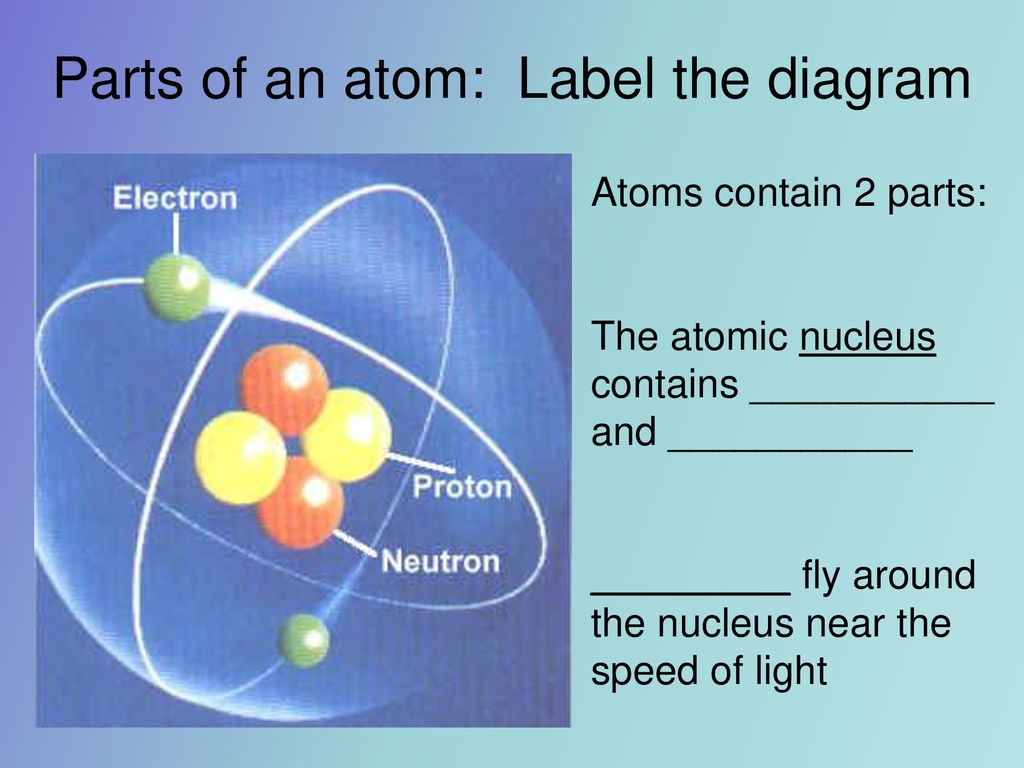
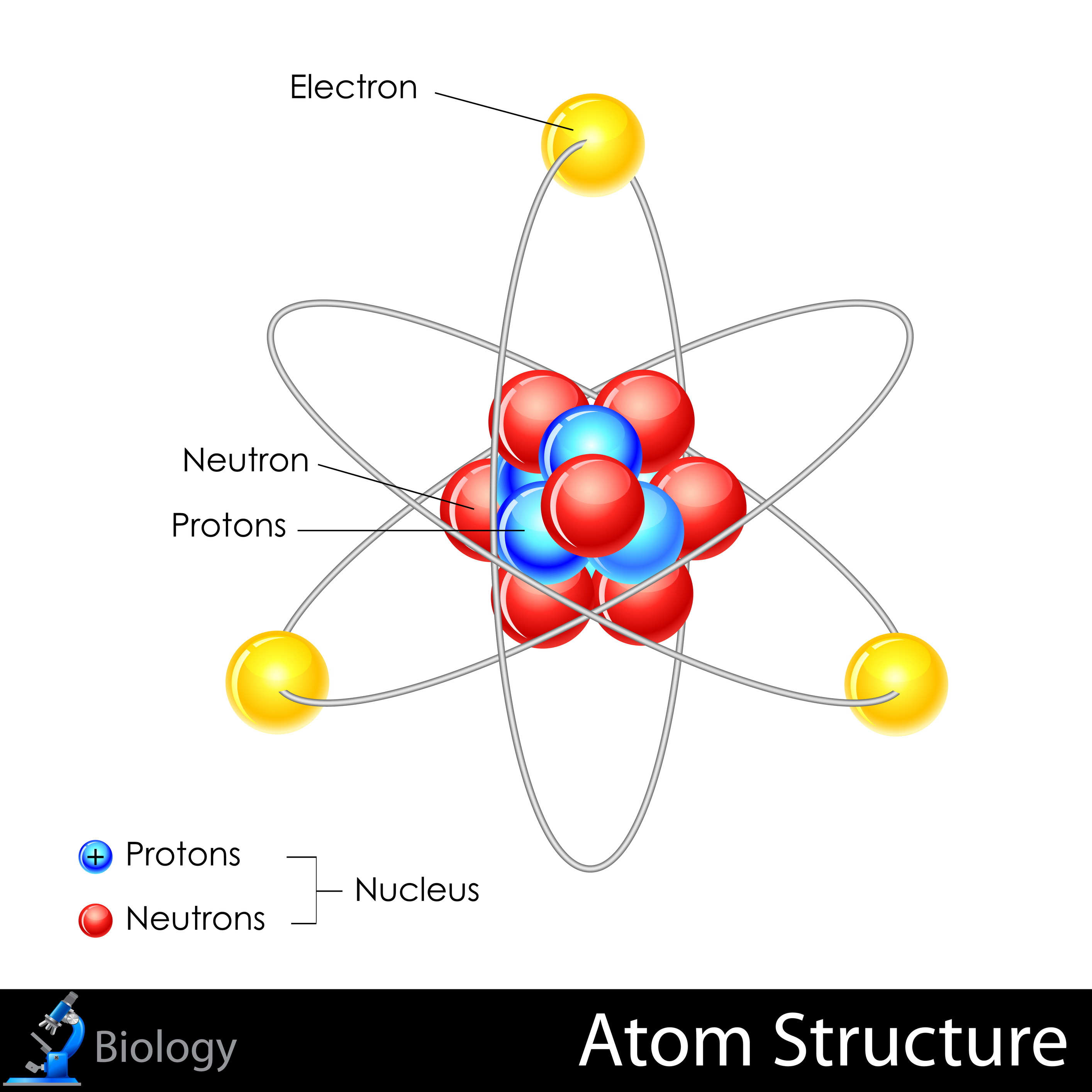
Label The Parts Of The Atom

Imagine peering into the heart of everything around you, shrinking down, down, down until the familiar world dissolves into a swarm of dancing particles. It's a realm of incredible energy and hidden structures, a universe contained within the seemingly solid objects we touch every day. We're diving into the world of atoms, the fundamental building blocks of matter, and embarking on a journey to understand their intricate parts.
This article aims to demystify the atom by clearly labeling its components – the protons, neutrons, and electrons – and explaining their roles in determining an element's properties. Understanding these basic building blocks is not merely an academic exercise; it's the foundation for understanding the world around us, from the chemical reactions that power our bodies to the technologies that shape our future.
A Glimpse into Atomic History
The concept of the atom isn't new. Ancient Greek philosophers, like Democritus, first proposed the idea of indivisible particles making up all matter centuries ago.
However, it wasn't until the 19th century that John Dalton's atomic theory provided a more scientific basis for this idea, suggesting that elements are composed of identical atoms and that chemical reactions involve the rearrangement of these atoms.
The 20th century brought revolutionary discoveries, revealing that the atom was not, in fact, indivisible, but composed of even smaller subatomic particles. Scientists like J.J. Thomson discovered the electron, leading to the "plum pudding" model of the atom.
The Nucleus: The Atom's Core
Ernest Rutherford's gold foil experiment dramatically changed our understanding. By firing alpha particles at a thin gold foil, he observed that some particles were deflected at large angles, suggesting a dense, positively charged center within the atom, which he named the nucleus.
The nucleus is composed of two types of particles: protons and neutrons. Protons carry a positive electrical charge, while neutrons are electrically neutral, contributing to the atom's mass but not its charge.
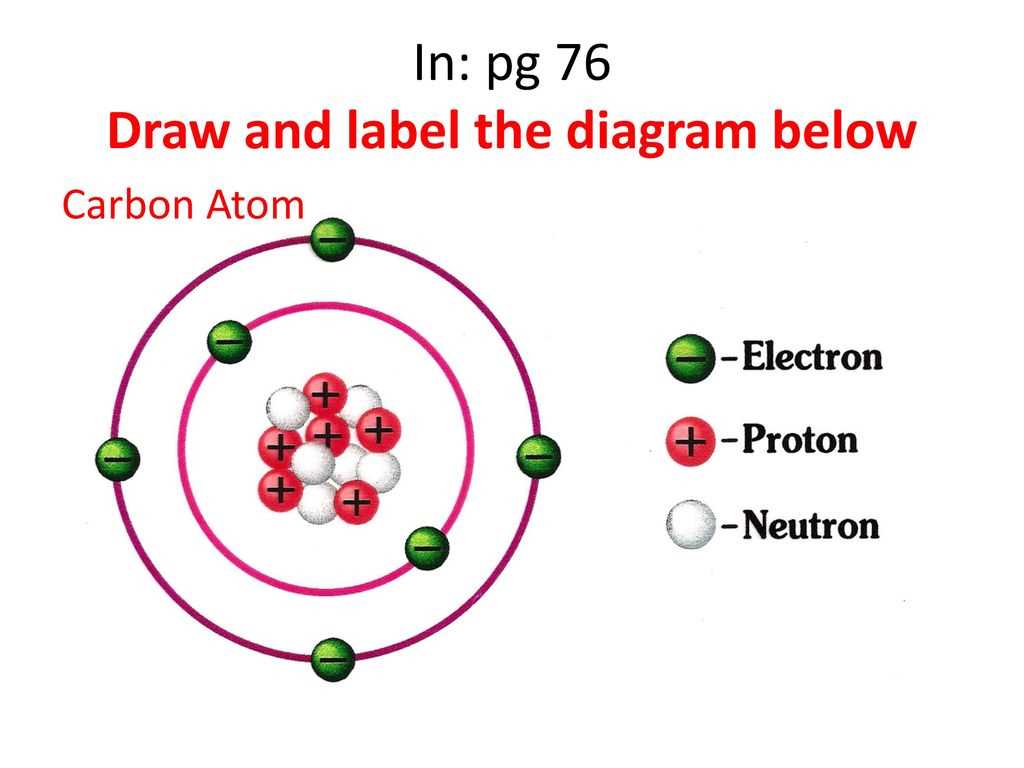
The number of protons in the nucleus, known as the atomic number, defines the element. For example, all atoms with one proton are hydrogen atoms, and all atoms with six protons are carbon atoms.
Electrons: Orbiting the Nucleus
Whizzing around the nucleus are electrons, negatively charged particles much smaller and lighter than protons and neutrons.
Unlike planets orbiting the sun in neat, predictable paths, electrons exist in specific energy levels or shells around the nucleus. These energy levels are quantized, meaning electrons can only exist at certain discrete energy values.
Electrons fill the innermost shells first before occupying the outer shells. The arrangement of electrons in these shells determines how an atom interacts with other atoms, forming chemical bonds and creating molecules.
Isotopes and Ions: Variations on a Theme
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes.
For example, carbon-12, carbon-13, and carbon-14 are all isotopes of carbon. They all have six protons, but they have six, seven, and eight neutrons, respectively.
Atoms can also gain or lose electrons, becoming electrically charged ions. Atoms that lose electrons become positively charged ions (cations), while atoms that gain electrons become negatively charged ions (anions). Ions are crucial in many chemical reactions, and are also essential in biological processes like nerve impulse transmission.
The Significance of Atomic Structure
Understanding the structure of the atom unlocks the secrets of the universe. The properties of elements, their reactivity, their ability to form different compounds, all stem from the arrangement of their protons, neutrons, and electrons.
From the medications we take to the materials we use to build our homes, everything relies on the interactions of atoms at a fundamental level.
Moreover, advancements in fields like nuclear energy and materials science are directly linked to our ability to manipulate and understand atomic structure.
Visualizing the Atom: Models and Metaphors
It's important to remember that the models we use to visualize the atom are simplified representations. The idea of electrons orbiting the nucleus like planets is a useful metaphor, but it's not entirely accurate.
Electrons behave according to the principles of quantum mechanics, exhibiting wave-particle duality. This means that they can act as both particles and waves, and their exact location at any given moment is probabilistic rather than precisely defined.
Modern models of the atom often depict electrons as existing in probability clouds, representing the regions where they are most likely to be found. These sophisticated models allow us to predict and explain the behavior of atoms with remarkable accuracy.
Looking Ahead: The Future of Atomic Research
Our journey into the atom is far from over. Scientists continue to probe the intricacies of atomic structure, seeking to understand the fundamental forces that govern the universe.
Research into new materials, advanced energy technologies, and quantum computing are all driving our exploration of the atomic realm.
As we continue to unlock the secrets of the atom, we can expect even more groundbreaking discoveries that will transform our world in unimaginable ways. The power within these tiny particles is truly boundless.
Understanding the atom, labeling its parts, and appreciating its significance is a key that unlocks our understanding of the universe itself. It’s a journey into the heart of matter, revealing the beauty and complexity that lie hidden within everything we see and touch. The atom is a testament to the power of human curiosity and the endless possibilities of scientific exploration.







/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)