Littmann Pediatric Stethoscope Engraving

Parents and medical professionals are urgently advised to inspect their Littmann Pediatric Stethoscopes immediately. Reports are surfacing regarding inconsistencies in the engraving depth, potentially leading to compromised identification and security risks.
This issue affects specifically the Littmann Pediatric Stethoscope models engraved between January 1, 2024, and July 31, 2024. The reduced engraving depth raises concerns about the longevity and legibility of personalized inscriptions, vital for preventing theft and ensuring proper ownership.
The Engraving Problem: What We Know
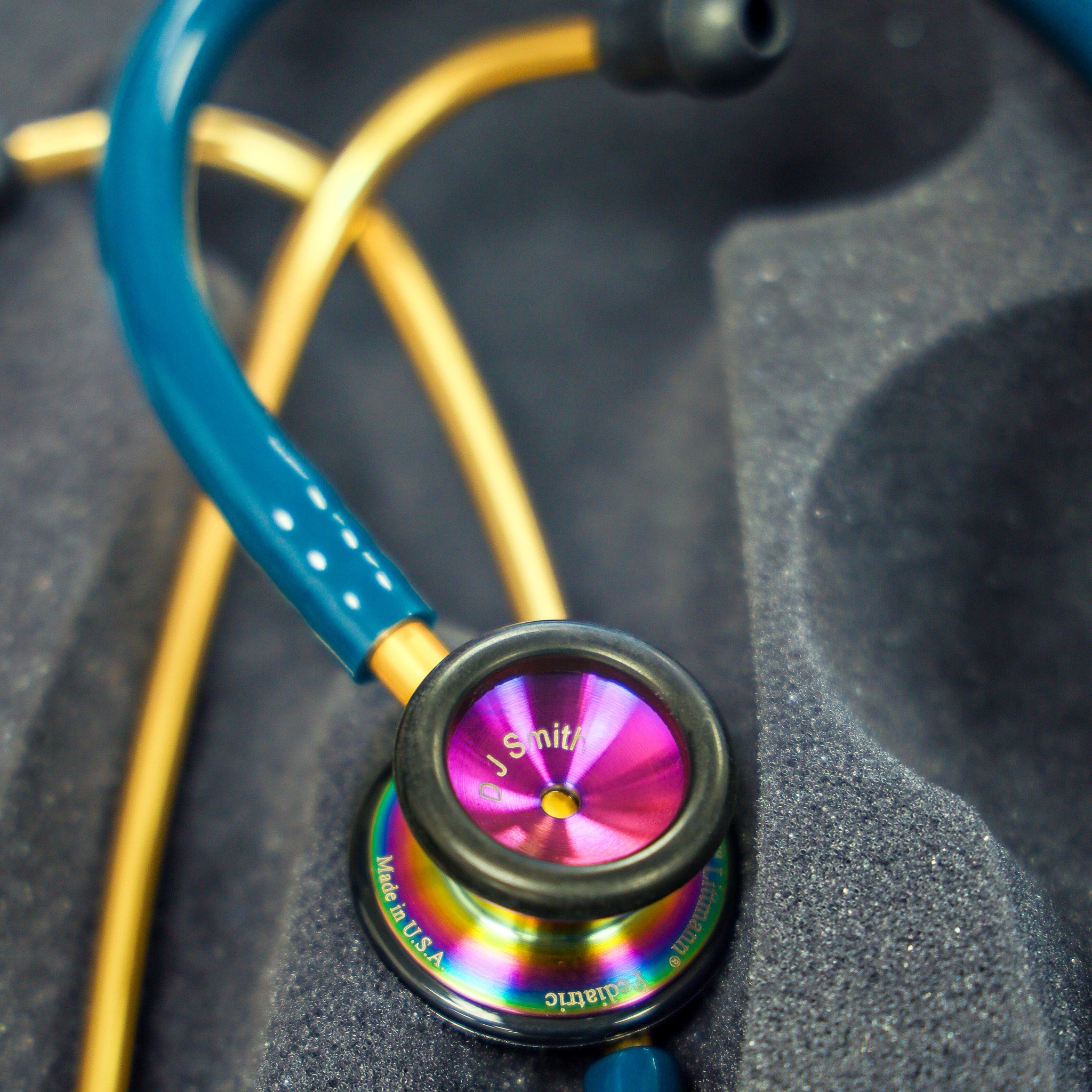
The issue centers around the depth of the laser engraving on the stethoscope's chestpiece. Instead of the standard depth, some stethoscopes produced during the specified period exhibit significantly shallower engravings.
This shallower engraving makes the personalized text or identification number prone to fading or wearing off with regular use. Consequently, it may become difficult to trace or identify the stethoscope if lost or stolen.
"We have received reports indicating a deviation from our standard engraving process," confirmed a 3M spokesperson. "We are taking these reports very seriously and are actively investigating the scope of the issue."
Affected Models and Production Dates
The affected models are strictly the Littmann Pediatric Stethoscopes. The production dates impacted are January 1, 2024, through July 31, 2024.
Stethoscopes purchased outside of this date range are not believed to be affected. Serial numbers can be cross-referenced with retailers to verify the production date.
How to Identify a Potentially Affected Stethoscope
Examine the engraving on the chestpiece closely. If the engraving appears faint, shallow, or easily rubbed off, it could be a sign of the defect.
Compare the engraving depth to that of other Littmann stethoscopes, if available. Contact 3M directly with the stethoscope's serial number for verification.
Immediate Actions and Recommendations
Individuals who purchased a Littmann Pediatric Stethoscope during the affected period should take the following steps. First, carefully inspect the engraving for depth and legibility.
Second, if the engraving is questionable, contact the retailer from whom the stethoscope was purchased. Third, directly contact 3M's customer support with the product's serial number.
Hospitals and clinics should initiate an internal review of their Littmann Pediatric Stethoscope inventory. Ensure proper tracking and identification measures are in place.
Next Steps and Ongoing Developments
3M is currently investigating the root cause of the engraving inconsistencies. They are expected to announce a corrective action plan within the next few weeks.
This plan may include options for stethoscope replacement or re-engraving. Further updates will be provided as they become available through 3M's official website and authorized retailers.
Stay vigilant and regularly check the engraving on your Littmann Pediatric Stethoscope. Immediate action is crucial to mitigate potential risks associated with this manufacturing defect.