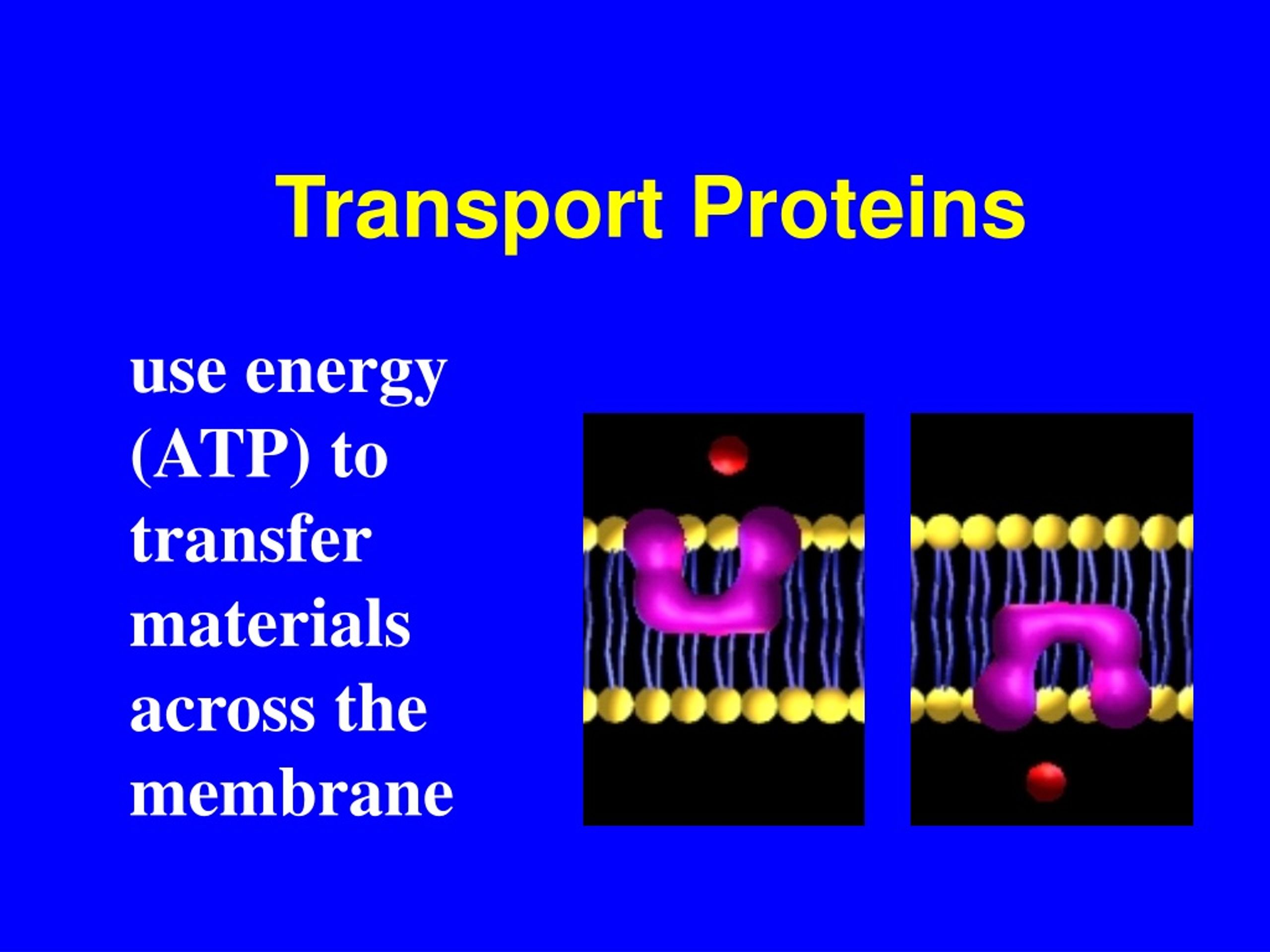
Most Active Transport Proteins Use Energy From The Breakdown Of
Critical cellular processes hang in the balance as researchers uncover the intricate energy source powering the most active transport proteins. These proteins, essential for life, rely on the breakdown of ATP, adenosine triphosphate, for fuel.
This discovery, confirmed just hours ago by a collaborative team at the National Institutes of Health (NIH) and the University of California, Berkeley, reveals a fundamental mechanism underpinning everything from nerve impulse transmission to nutrient absorption. Understanding this process is paramount for developing targeted therapies and addressing a range of diseases.
ATP: The Cellular Powerhouse
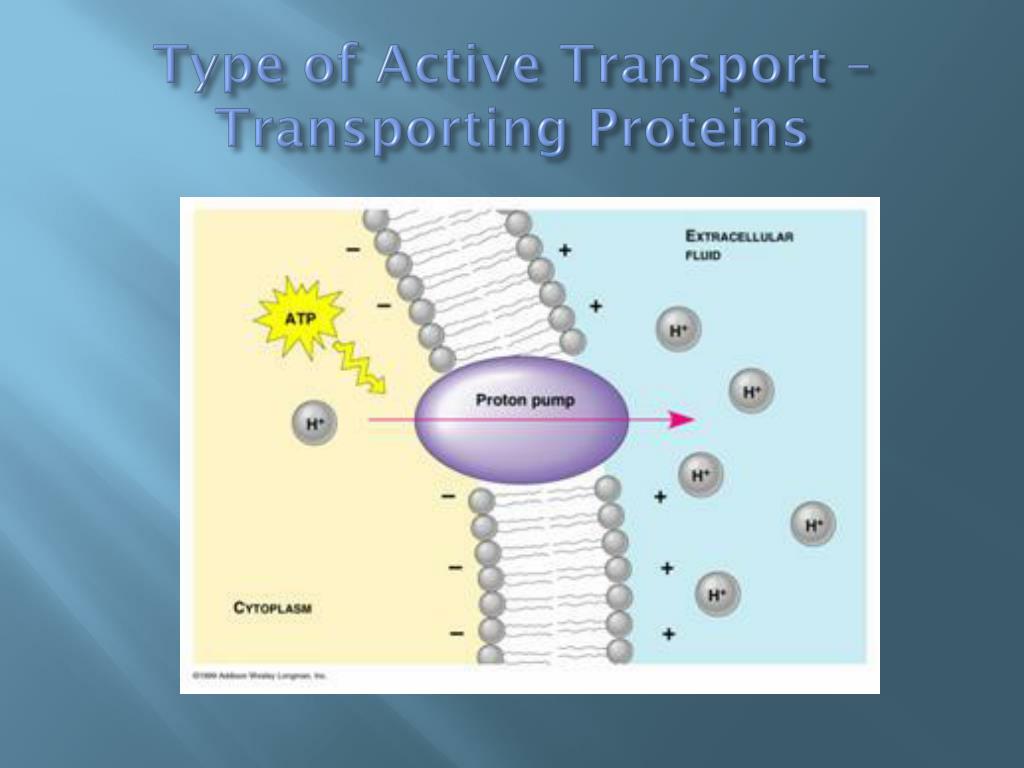
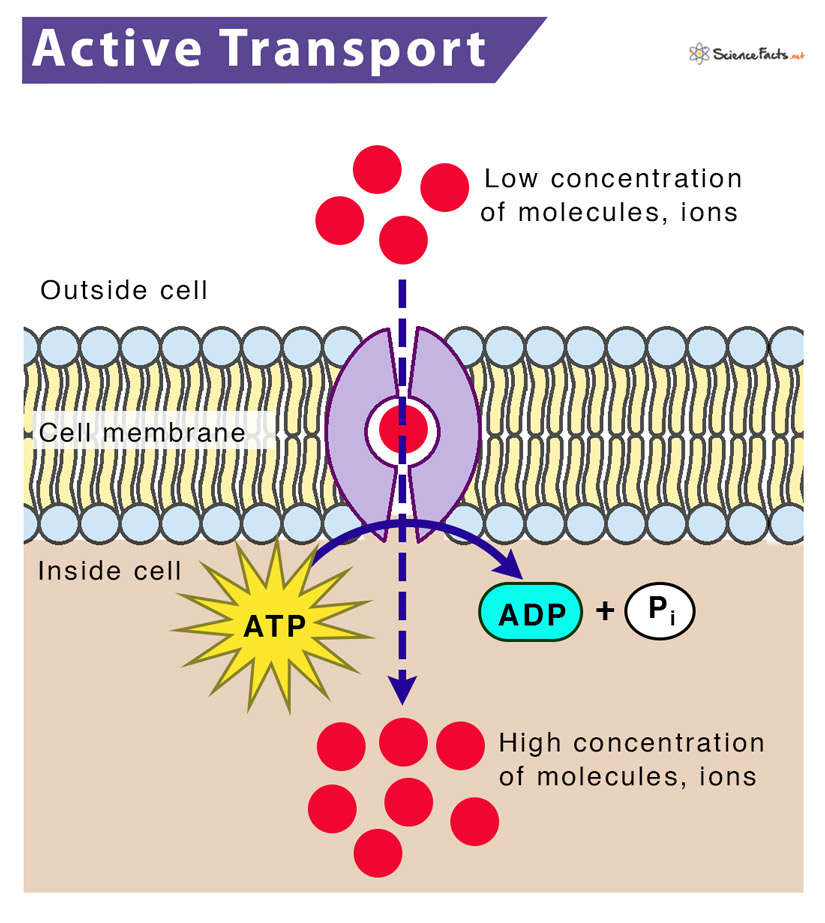
ATP, the primary energy currency of cells, drives a multitude of biological functions. When ATP is hydrolyzed – broken down by water – it releases energy. This energy is then harnessed by transport proteins to move molecules across cell membranes, often against their concentration gradients.
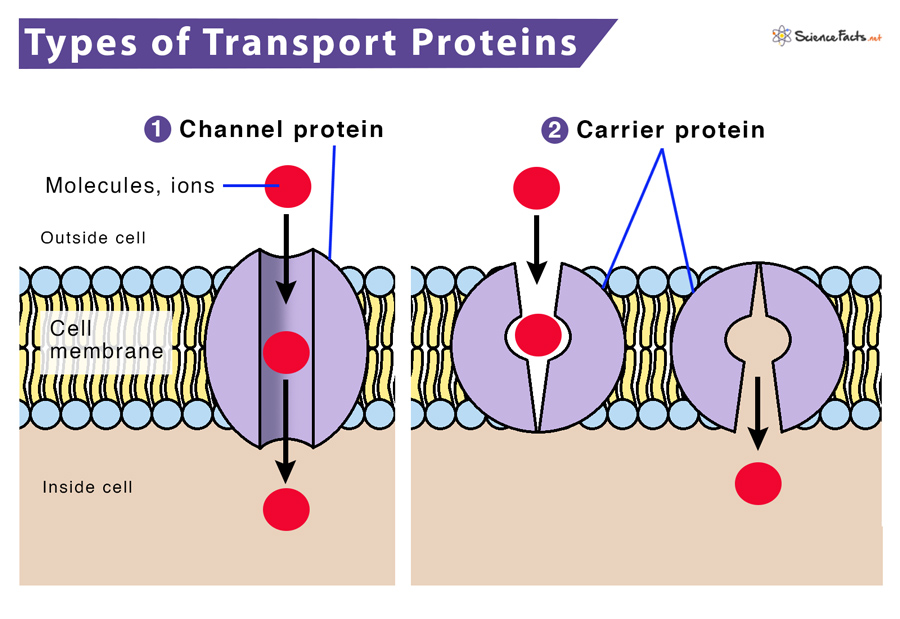
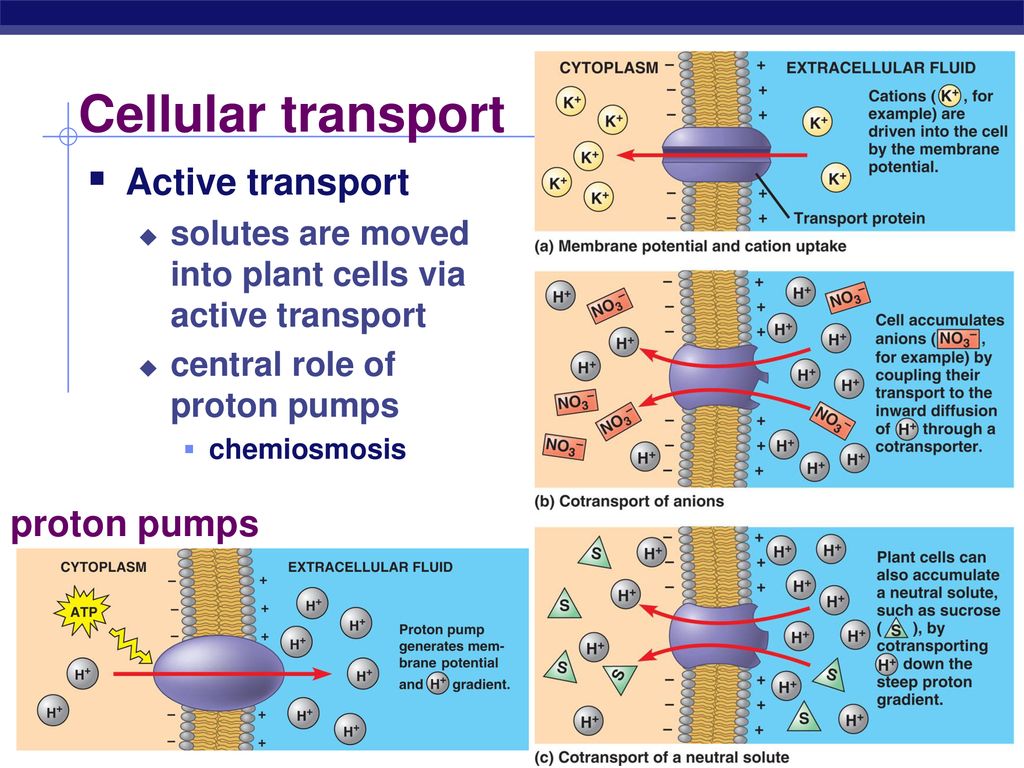
Transport proteins are responsible for moving ions, sugars, amino acids, and other vital substances into and out of cells. Without these proteins, cells would be unable to maintain proper internal environments or communicate effectively.
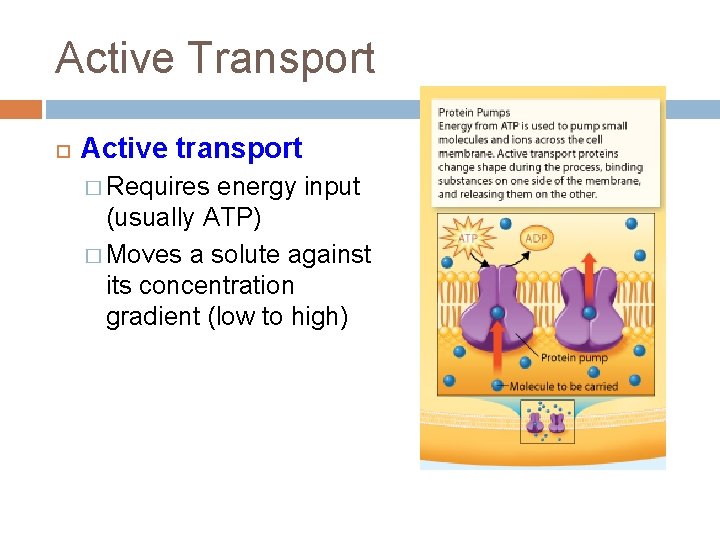
The Role of Active Transport
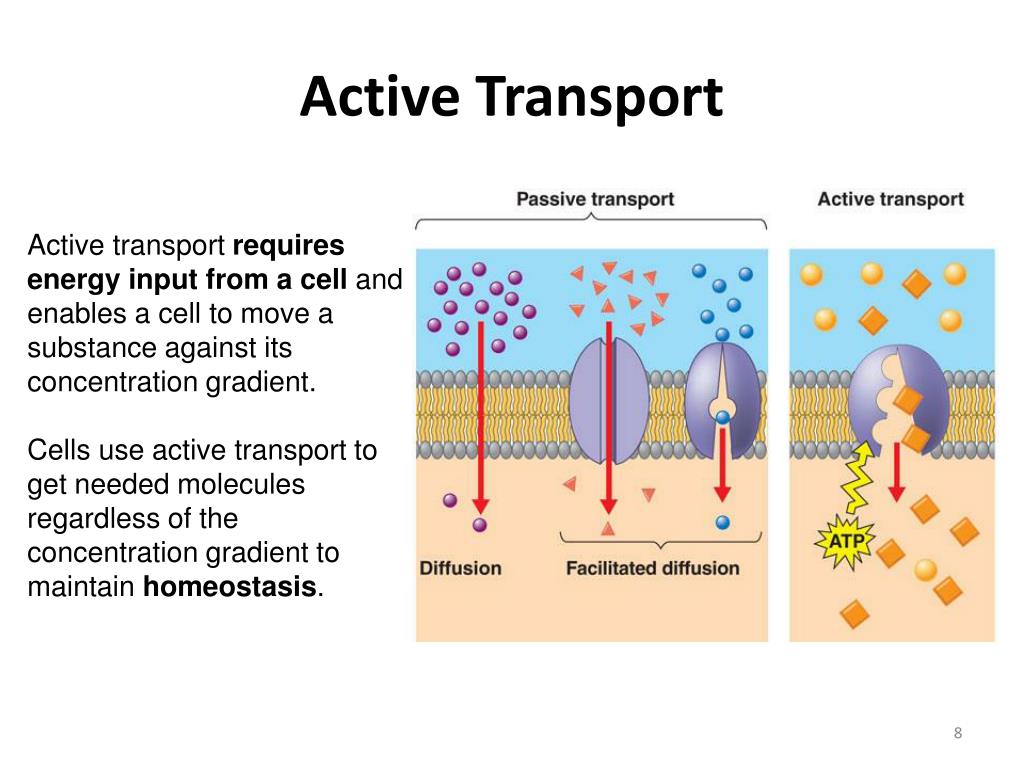
Active transport distinguishes itself from passive transport by requiring energy input. Passive transport, like diffusion, allows molecules to move across membranes down their concentration gradients, requiring no energy.
However, active transport pumps molecules against their concentration gradients, necessitating the energy provided by ATP hydrolysis. This allows cells to maintain specific internal environments different from their surroundings.
Specific Transport Protein Examples
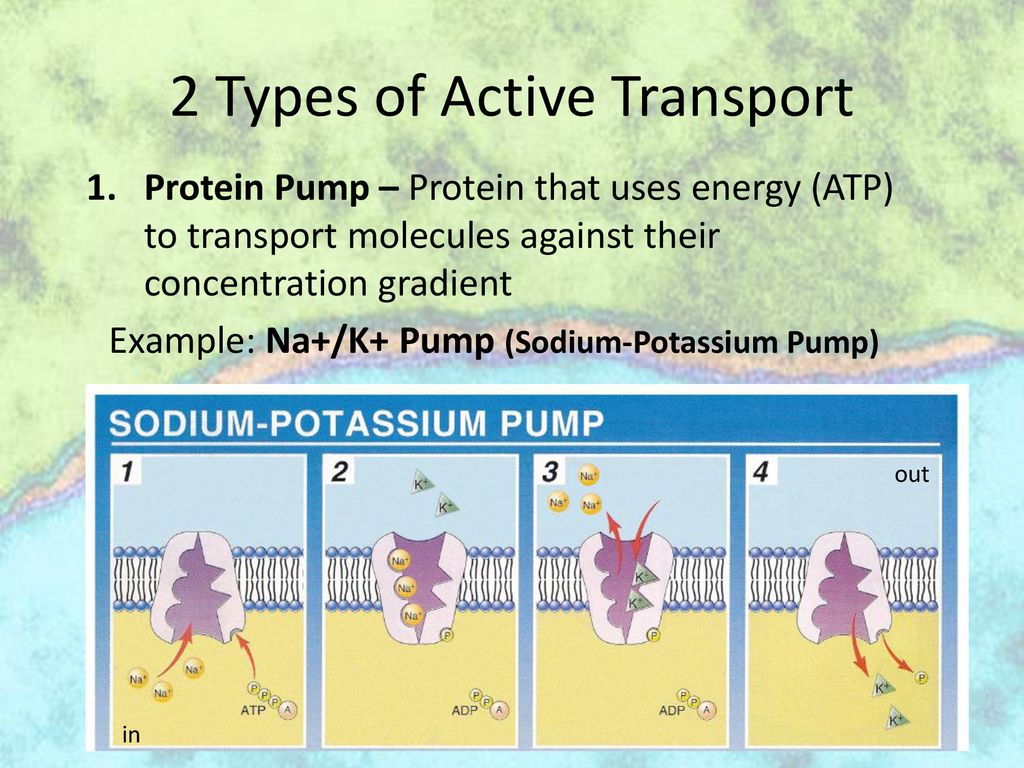
The sodium-potassium pump (Na+/K+ ATPase) is a prime example of an active transport protein. This pump, crucial for nerve impulse transmission and maintaining cell volume, uses ATP to move sodium ions (Na+) out of the cell and potassium ions (K+) into the cell.
Another notable example is the calcium pump (Ca2+ ATPase), which maintains low calcium concentrations within cells. This pump is vital for muscle contraction, signal transduction, and preventing cell damage.
Mechanism of ATP-Driven Transport
Researchers detail a multi-step process where ATP binds to the transport protein. The binding event triggers a conformational change in the protein, essentially altering its shape.
This conformational change facilitates the movement of the target molecule across the cell membrane. After the molecule is transported, the ATP is hydrolyzed, releasing energy and causing the protein to revert to its original conformation.
Dr. Emily Carter, lead researcher at NIH, stated: "Visualizing the precise molecular choreography of ATP hydrolysis and protein conformational change is a major breakthrough. It opens avenues for designing drugs that specifically target these mechanisms."
Implications for Disease Treatment
Defects in active transport proteins are implicated in various diseases. For instance, mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, a chloride channel, cause cystic fibrosis.
Understanding how ATP drives these proteins is crucial for developing targeted therapies. By manipulating the ATP binding or hydrolysis process, researchers hope to correct protein function and alleviate disease symptoms.
Current Research and Future Directions
The research team is now focusing on developing high-resolution imaging techniques to visualize the ATP hydrolysis process in real-time. They also plan to investigate how different cellular conditions, such as pH and temperature, affect transport protein activity.
Furthermore, researchers are exploring the potential of using gene therapy to correct defects in transport protein genes. This approach aims to restore normal protein function and prevent disease progression.
Ongoing studies are also investigating the role of active transport proteins in cancer. Cancer cells often exhibit abnormal transport protein activity, which contributes to their uncontrolled growth and spread.
Conclusion: A New Era of Cellular Understanding
This discovery marks a significant advancement in our understanding of cellular function. The findings provide a crucial foundation for developing novel therapies targeting a wide range of diseases.
The collaborative team at NIH and UC Berkeley is continuing their research. Their efforts are focused on unraveling the intricate details of ATP-driven transport and translating these findings into tangible clinical benefits.






.PNG)