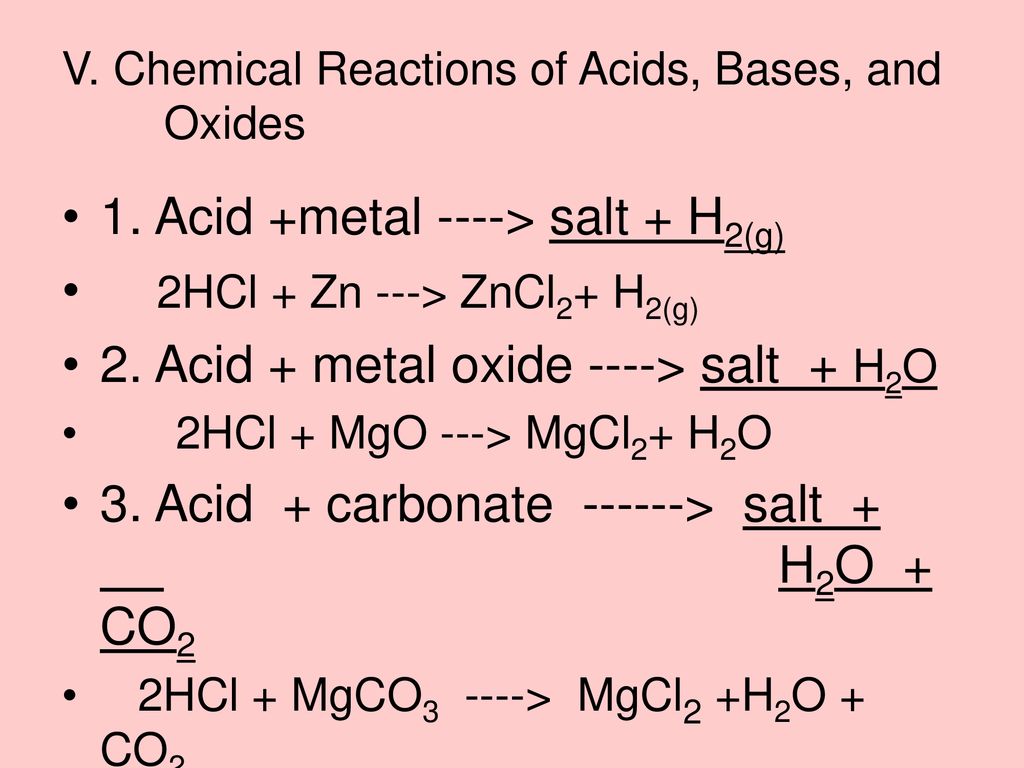
Mutual Reaction Of Acid And Base Is Called

Imagine a bustling high school chemistry lab, the air thick with the scent of diluted vinegar and something vaguely citrusy. Beakers bubble gently on hotplates, and the rhythmic drip of a burette fills the room. A hush falls as a student carefully adds a clear liquid to a pink solution. The color vanishes, replaced by…nothing. A collective gasp, a shared moment of understanding washes over the class. In that instant, a complex chemical dance, a fundamental principle of nature, has played out before their eyes.
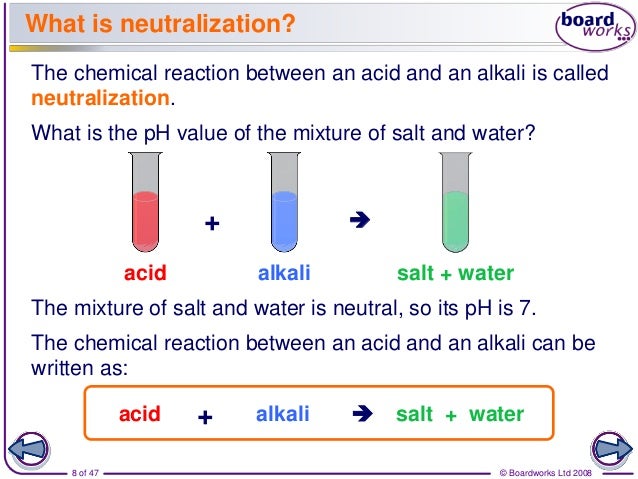
This simple yet profound demonstration illustrates the mutual reaction of an acid and a base, a process we call neutralization. It’s a cornerstone of chemistry, impacting everything from the food we eat to the environment we inhabit. Understanding neutralization isn't just about balancing equations; it's about grasping the interactions that shape our world.
Delving into Acids and Bases
To truly appreciate neutralization, we must first understand the actors involved: acids and bases. For centuries, scientists observed that acids tasted sour and could corrode metals. Bases, on the other hand, felt slippery and could neutralize the effects of acids.
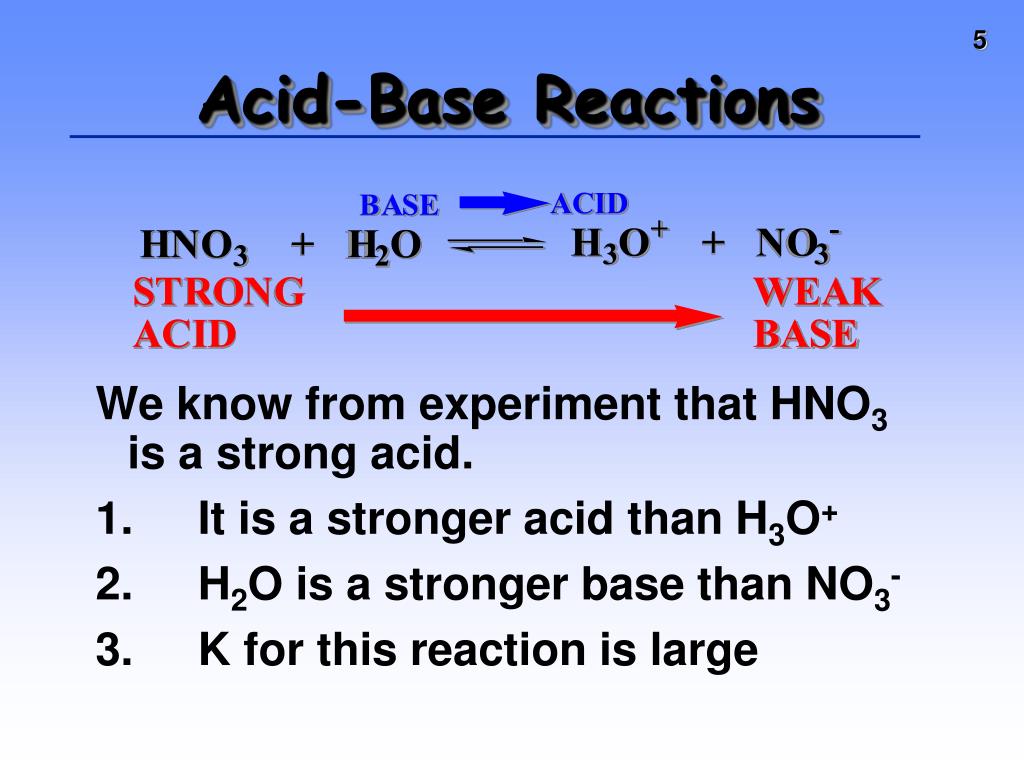
Svante Arrhenius, a Swedish scientist, revolutionized our understanding in the late 19th century. He proposed that acids are substances that increase the concentration of hydrogen ions (H+) in water, while bases increase the concentration of hydroxide ions (OH-).
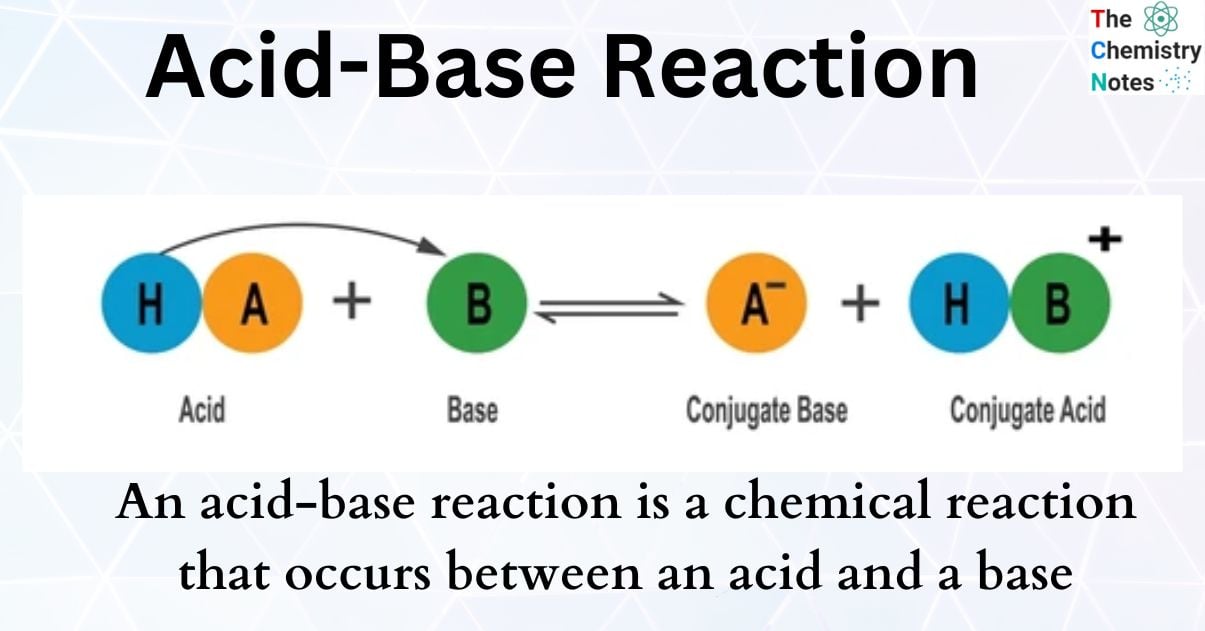
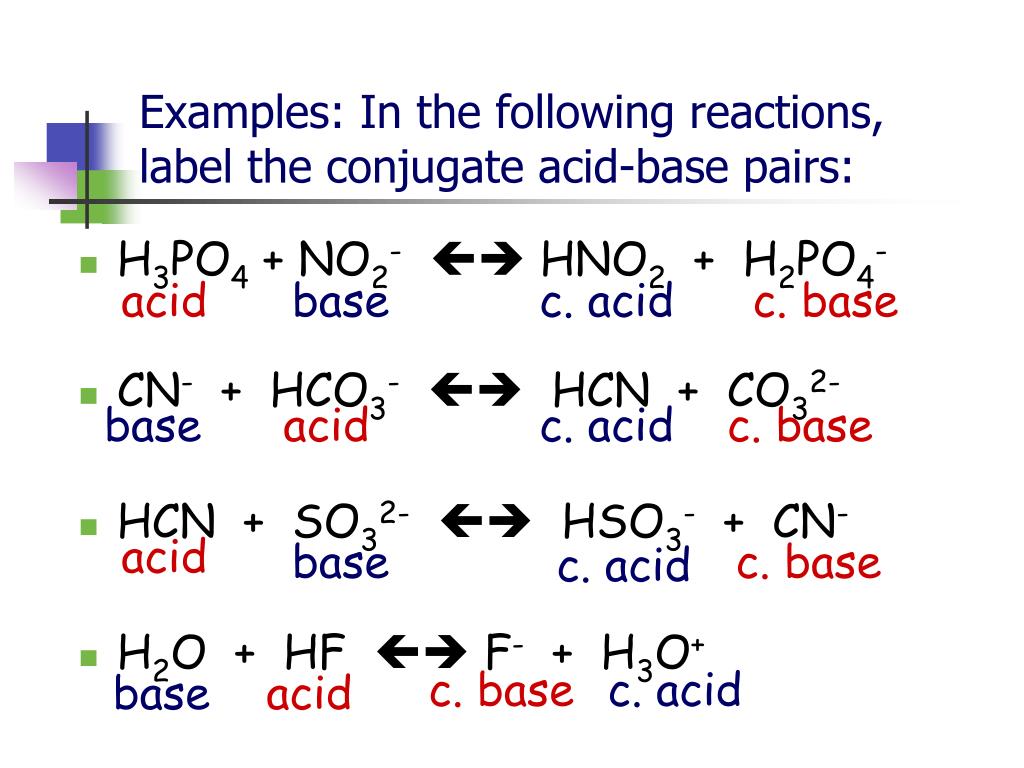
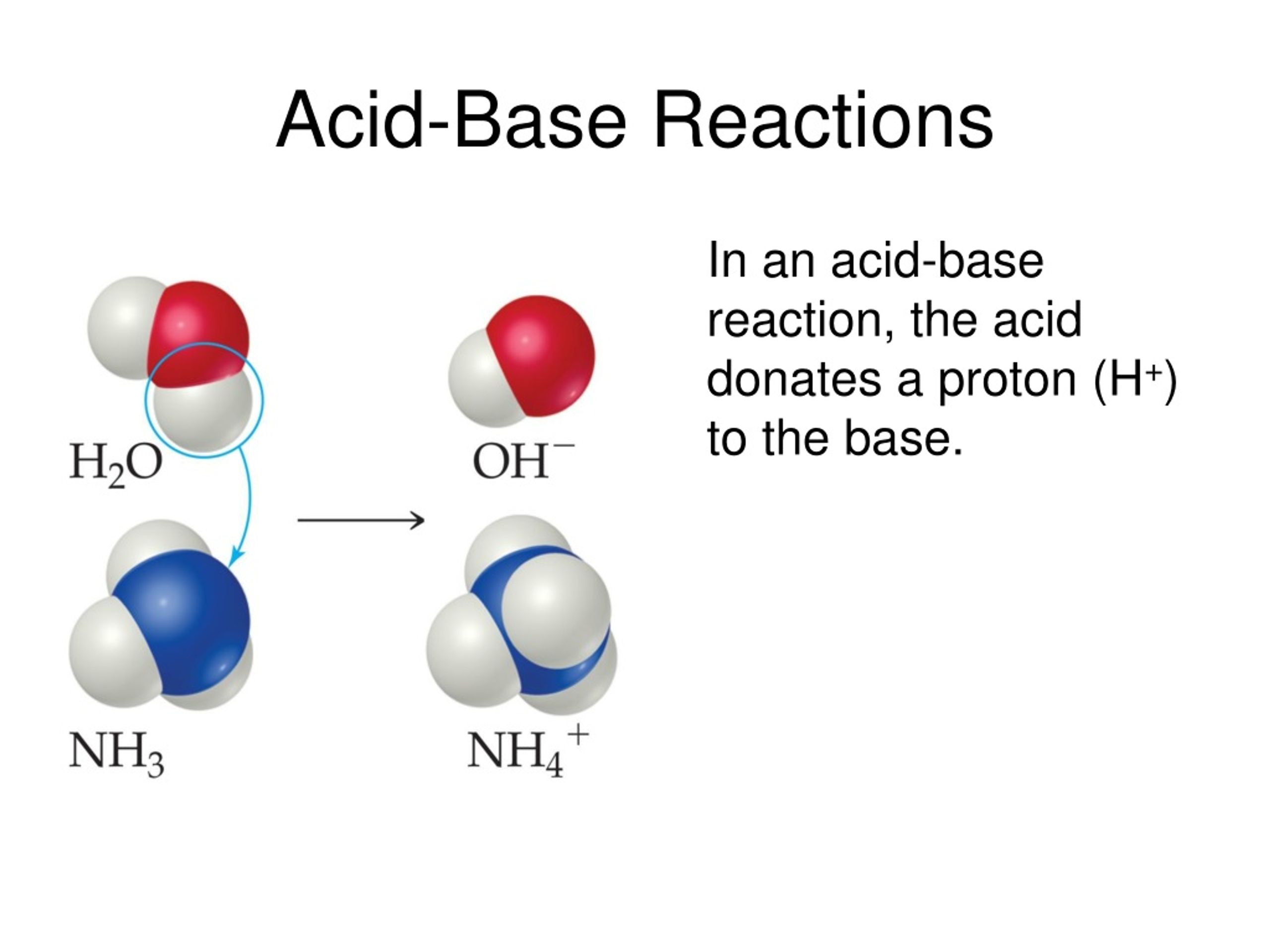
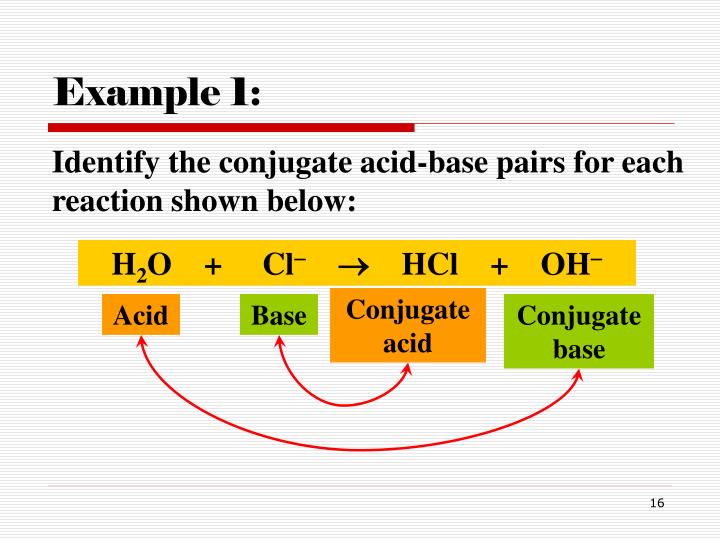
Later, scientists developed broader definitions. The Bronsted-Lowry definition defines an acid as a proton (H+) donor and a base as a proton acceptor. The Lewis definition even further broadens the scope, defining acids as electron-pair acceptors and bases as electron-pair donors.
The Dance of Neutralization
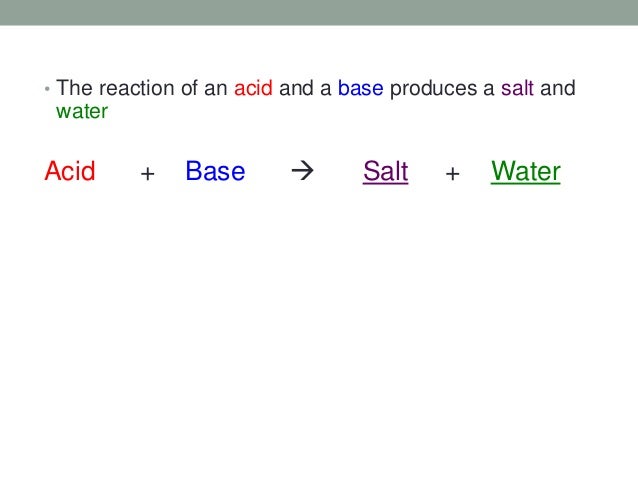
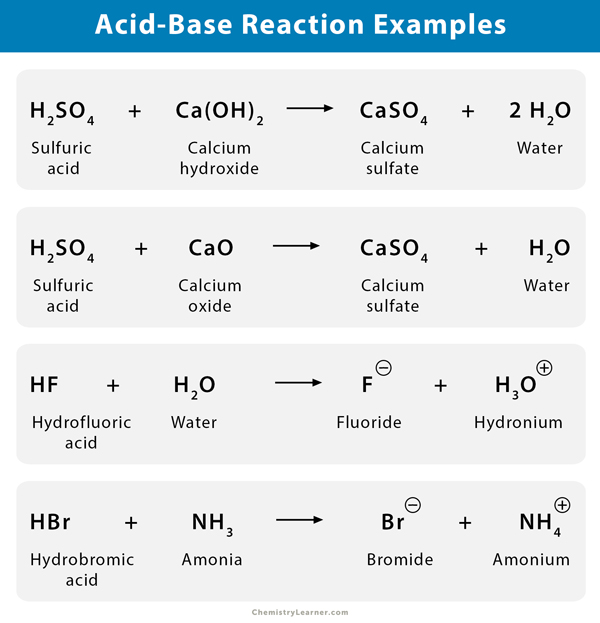
Neutralization is the reaction that occurs when an acid and a base are mixed. The H+ ions from the acid react with the OH- ions from the base to form water (H2O). This process effectively cancels out the acidic and basic properties of the original substances.
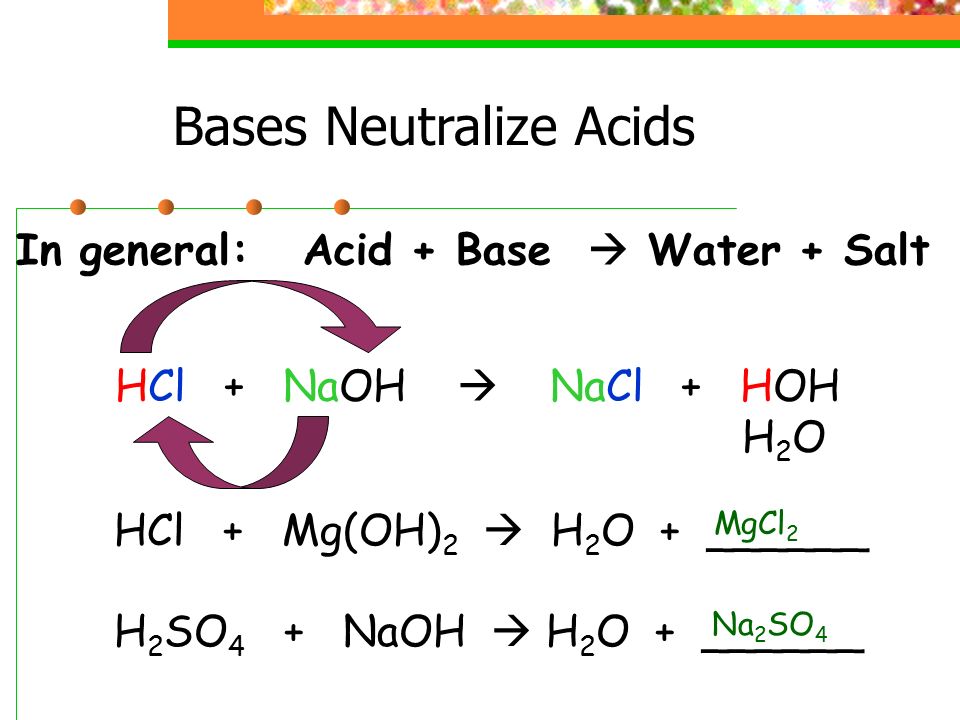
The classic example is the reaction between hydrochloric acid (HCl), a strong acid, and sodium hydroxide (NaOH), a strong base. The reaction produces sodium chloride (NaCl), common table salt, and water.
The overall equation is: HCl + NaOH → NaCl + H2O. Note that besides water, a salt is also formed. Salt is an ionic compound composed of a cation (positive ion) and an anion (negative ion).
The Significance of Neutralization
Neutralization reactions are not confined to laboratory beakers. They play crucial roles in numerous natural and industrial processes. Our bodies constantly use neutralization to maintain a stable pH level in our blood and digestive system.
Antacids, for example, utilize neutralization to relieve heartburn. They contain bases, like magnesium hydroxide, which neutralize excess stomach acid, providing relief.
In agriculture, farmers use lime (calcium carbonate) to neutralize acidic soils, creating a more favorable environment for crops. This helps plants absorb nutrients more effectively, boosting yields.
Industrial wastewater treatment also relies heavily on neutralization. Before releasing wastewater into the environment, companies often need to neutralize it to prevent pollution and harm to aquatic life.
Looking Ahead
The simple act of mixing an acid and a base unlocks a world of possibilities. As our understanding of chemistry evolves, so too will our ability to harness the power of neutralization for new and innovative applications.
From developing new medications to improving environmental sustainability, the principles of neutralization will continue to shape our future. It began in a lab, but now it's part of our world.
Next time you see a vinegar bottle, think about the amazing chemistry that happens when acids and bases come together. It's a reminder that even the simplest reactions can have a profound impact.


..jpg)
.PNG)

![Mutual Reaction Of Acid And Base Is Called How do Acids and Bases react with Metals? [with Examples] - Teachoo](https://d1avenlh0i1xmr.cloudfront.net/a05447d8-637a-4e50-8d67-d732eee7c48d/how-do-acids-and-bases-react-with-metals---teachoo.jpg)