No Amino Acid Molecule By Itself

Groundbreaking research has revealed a startling truth: amino acid molecules, the building blocks of proteins and life itself, cannot exist independently in nature.
This paradigm shift challenges long-held assumptions about the origins of life and the fundamental chemistry that underpins biology.
Discovery Details
The findings, published in the latest issue of Nature Chemistry, stem from a series of sophisticated experiments conducted at the Max Planck Institute for Biophysical Chemistry in Göttingen, Germany.
The research team, led by Dr. Elena Rossi, utilized advanced mass spectrometry and computational modeling techniques to meticulously analyze the behavior of individual amino acid molecules in various simulated environments.
Contrary to expectations, the team discovered that free-floating amino acids are inherently unstable and rapidly degrade without the stabilizing presence of other molecules, specifically water or other amino acids.
Methodology
Dr. Rossi's team created a near-vacuum environment to isolate individual amino acid molecules.
They then introduced minute quantities of water vapor and observed a dramatic increase in the amino acids' stability.
Further experiments involved clustering amino acids together, demonstrating a similar stabilizing effect and highlighting the importance of molecular interactions.
The simulations corroborated these findings, revealing that the inherent polarity and reactivity of amino acids necessitate a surrounding environment to neutralize their instability.
Implications for the Origin of Life
This discovery forces a reevaluation of theories concerning the abiogenesis, the process by which life arises from non-living matter.
Traditional models often depict amino acids spontaneously forming in a primordial soup and subsequently assembling into larger proteins.
This new research suggests that such a scenario is highly improbable without the pre-existence of a structured environment capable of supporting and stabilizing individual amino acid molecules.
Expert Commentary
"Our findings indicate that the origin of life might have required a more complex and constrained environment than previously imagined," stated Dr. Rossi in a press conference.
Professor James Carter, a leading expert in prebiotic chemistry at Harvard University, commented, "This is a significant and potentially revolutionary finding that will undoubtedly reshape our understanding of how life began."
"The implications are profound and suggest that we need to rethink the models we use to study the emergence of biological molecules," added Professor Carter.
Where and When
The research was conducted over a period of three years at the Max Planck Institute for Biophysical Chemistry, culminating in the publication of the findings on October 26, 2023.
The Institute is renowned for its cutting-edge research in biophysics and structural biology.
The experiments and simulations utilized state-of-the-art equipment and software, ensuring the accuracy and reliability of the results.
Impact on Future Research
The discovery is already prompting new lines of inquiry into the role of minerals, membranes, and other environmental factors in the early stages of life.
Researchers are now focusing on investigating the potential of clay minerals and other inorganic materials to provide a stable platform for amino acid assembly.
Furthermore, the findings highlight the need for more sophisticated computational models that accurately capture the complex interactions between amino acids and their surrounding environment.
Who is Involved
The core research team consisted of Dr. Elena Rossi, Dr. Markus Schmidt, and Dr. Anya Petrova, all from the Max Planck Institute.
The project also involved collaborations with researchers from the University of California, Berkeley, who provided expertise in computational modeling.
Funding for the research was provided by the German Research Foundation and the European Research Council.
Next Steps
Dr. Rossi's team plans to investigate the behavior of amino acids in more complex environments, including those that mimic the conditions found in hydrothermal vents and other extreme environments.
The team also aims to explore the role of chirality, the property of molecules existing in left-handed and right-handed forms, in the stabilization of amino acid clusters.
These ongoing investigations promise to further refine our understanding of the origins of life and the fundamental chemistry that underpins all biological processes.
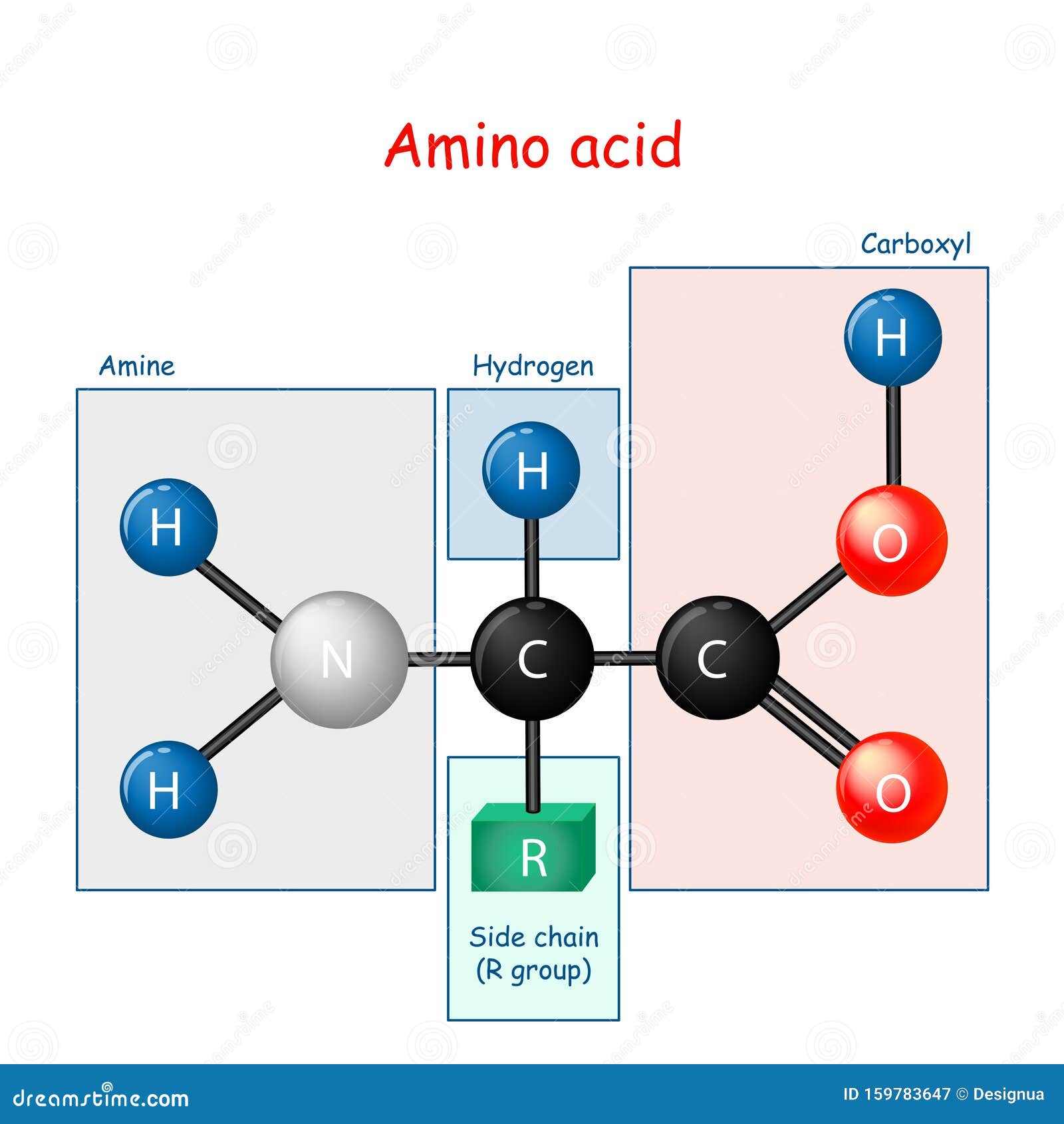

![No Amino Acid Molecule By Itself [DIAGRAM] Diagram Amino Acid - MYDIAGRAM.ONLINE](https://thumbs.dreamstime.com/z/c-h-no-valine-amino-acid-molecule-d-isolated-white-64906304.jpg)

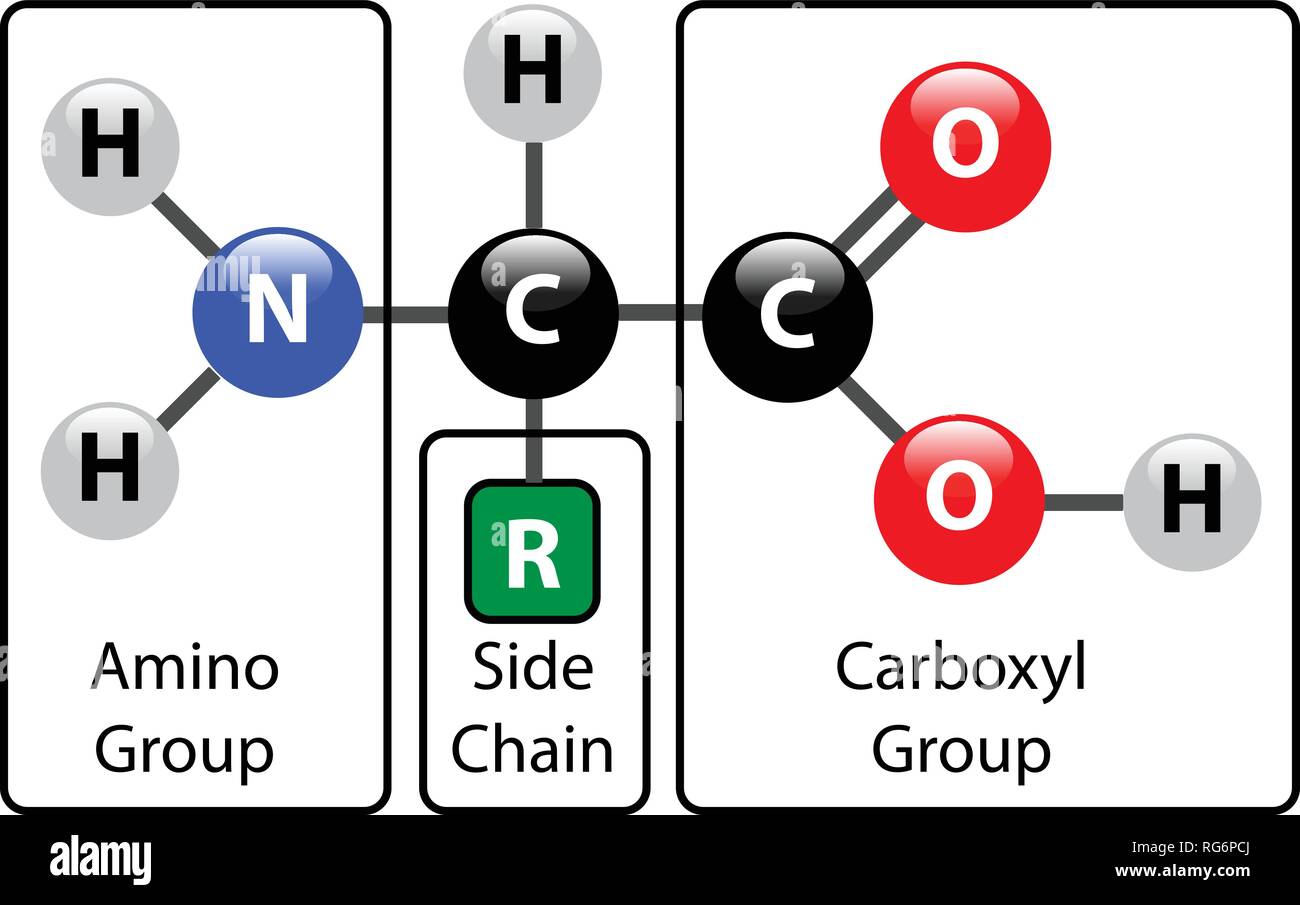
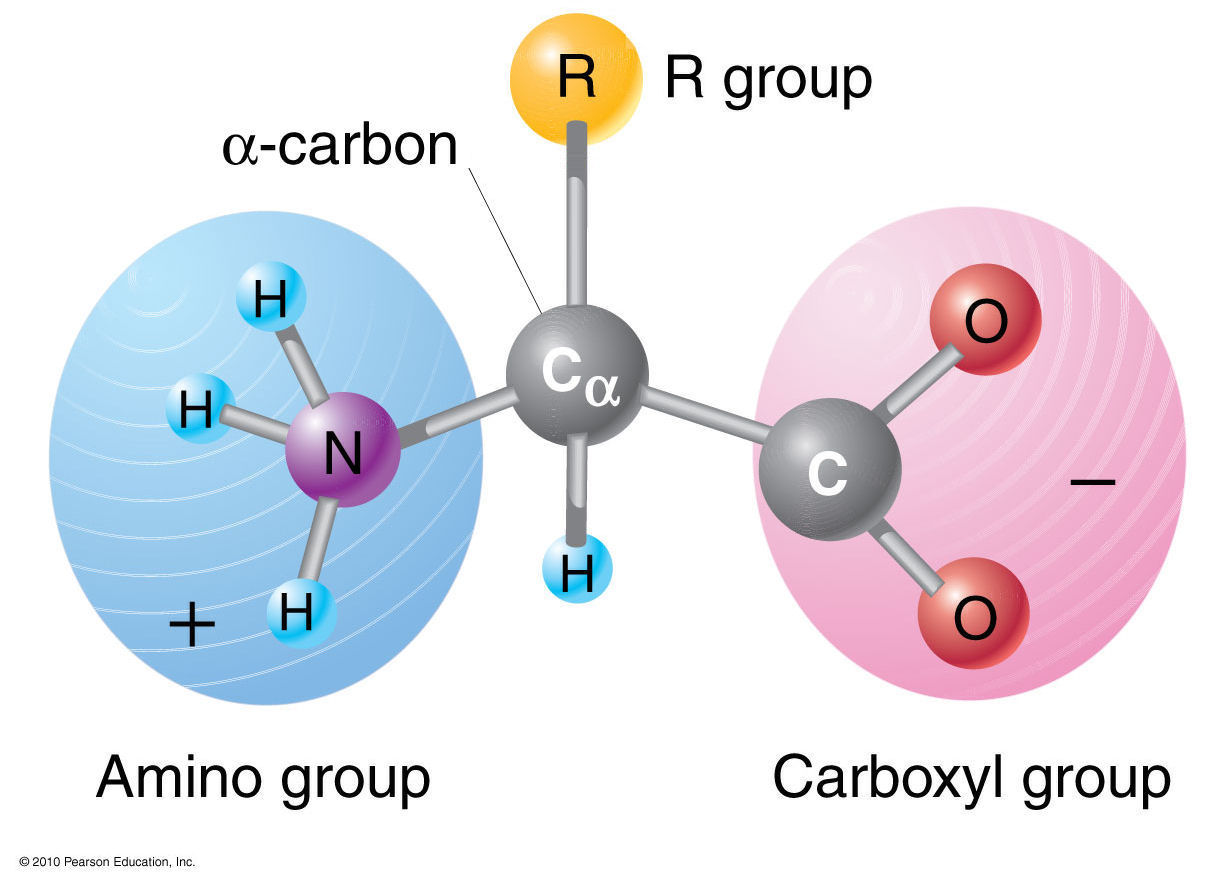



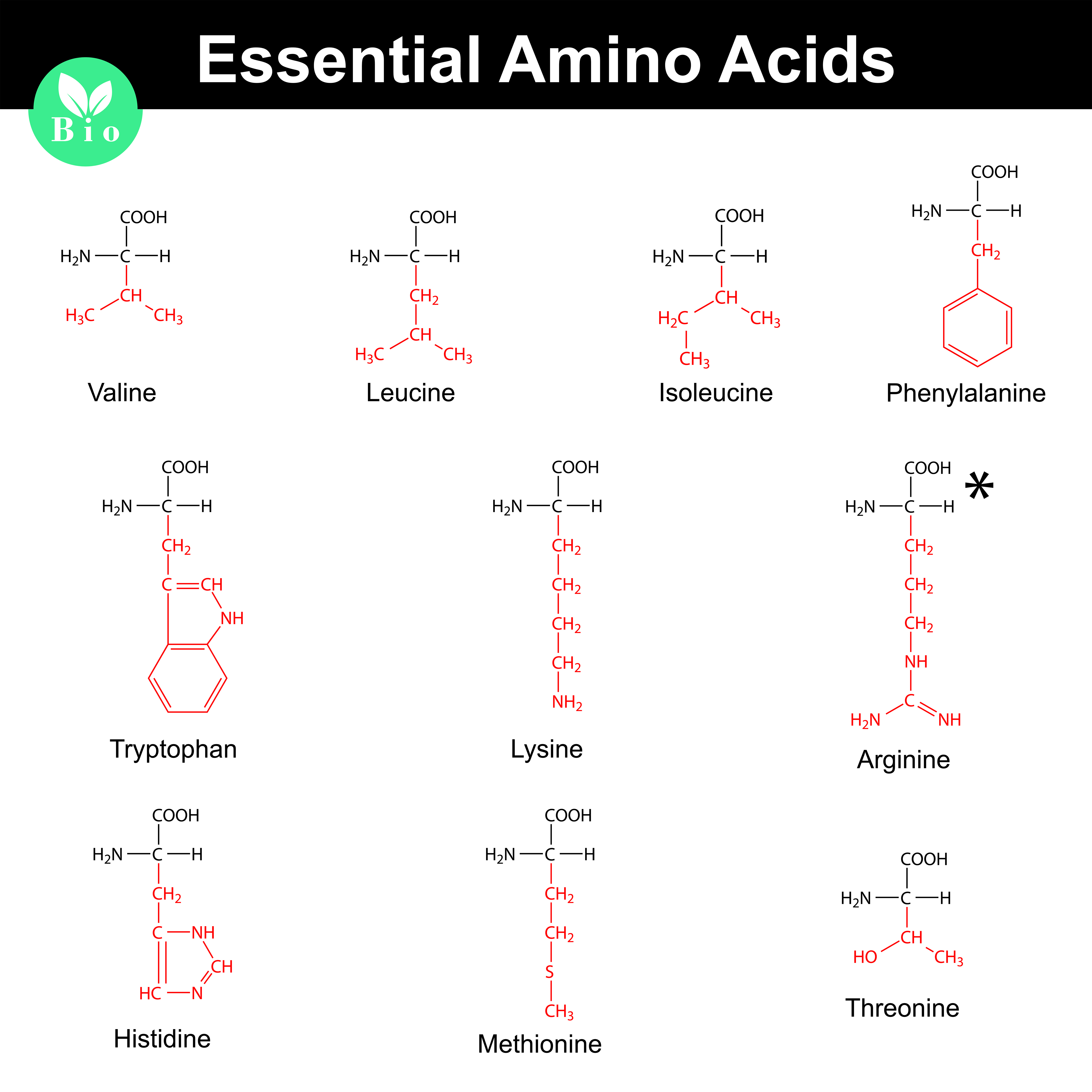



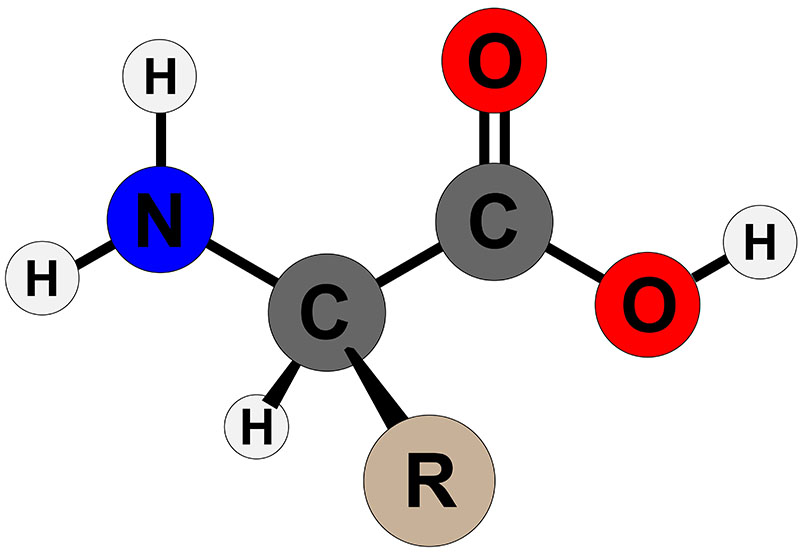
:max_bytes(150000):strip_icc()/amino_acid_structure-58c9599d3df78c353c9b5d2e.jpg)


