Patient Information Includes All Of The Following Except The
The security and accessibility of patient information have become increasingly critical in modern healthcare. Recent discussions surrounding data breaches and evolving regulations highlight the need to define clear boundaries of what constitutes protected health information (PHI). A growing concern is understanding exactly what patient data is, and perhaps more importantly, what it *isn't*, encompassed under legal safeguards.
At the heart of this debate lies a deceptively simple question: what information is protected under laws like HIPAA and GDPR? The answer to this question, often more complex than initially assumed, has far-reaching implications for patient privacy, data security, and the future of healthcare technology. This article examines the scope of patient information protection, clarifies which types of data fall outside the traditional definition, and explores the potential ramifications of these exclusions.
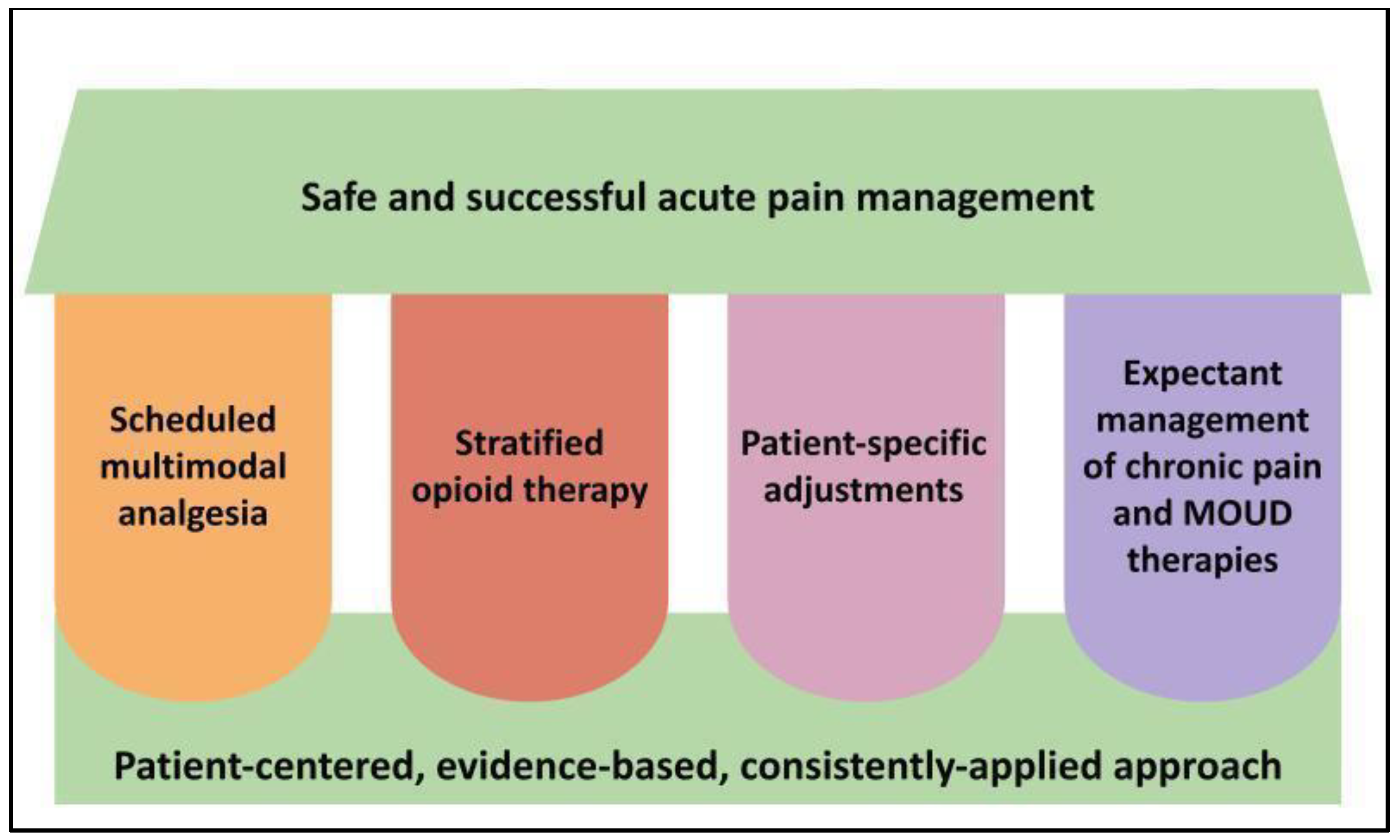
Understanding Protected Health Information (PHI)
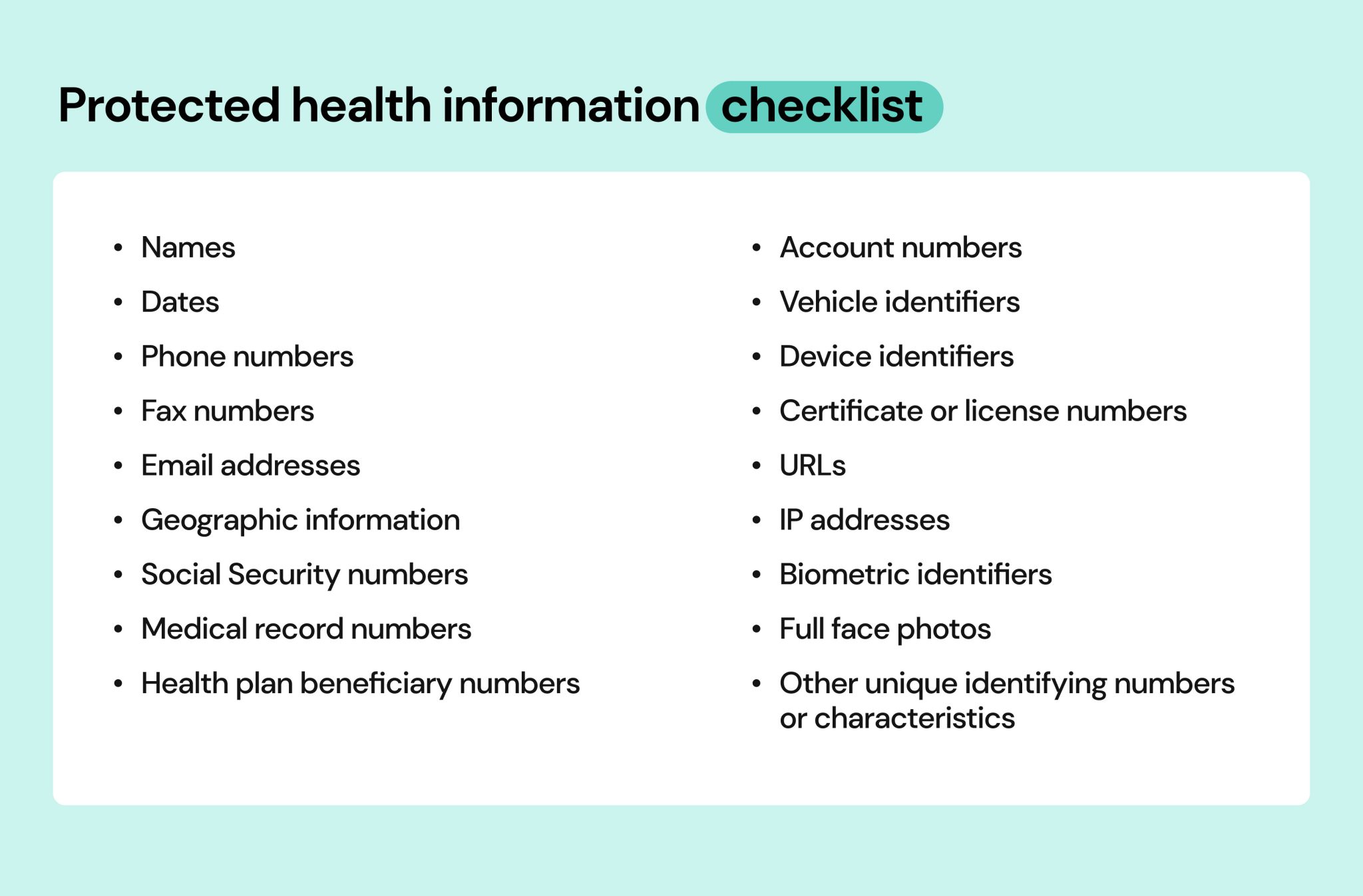
Protected Health Information (PHI) is any individually identifiable health information that is transmitted or maintained in any form or medium. This data must be created or received by a covered entity, such as a doctor's office, hospital, health insurer, or healthcare clearinghouse. The key element is the data's link to a specific individual.
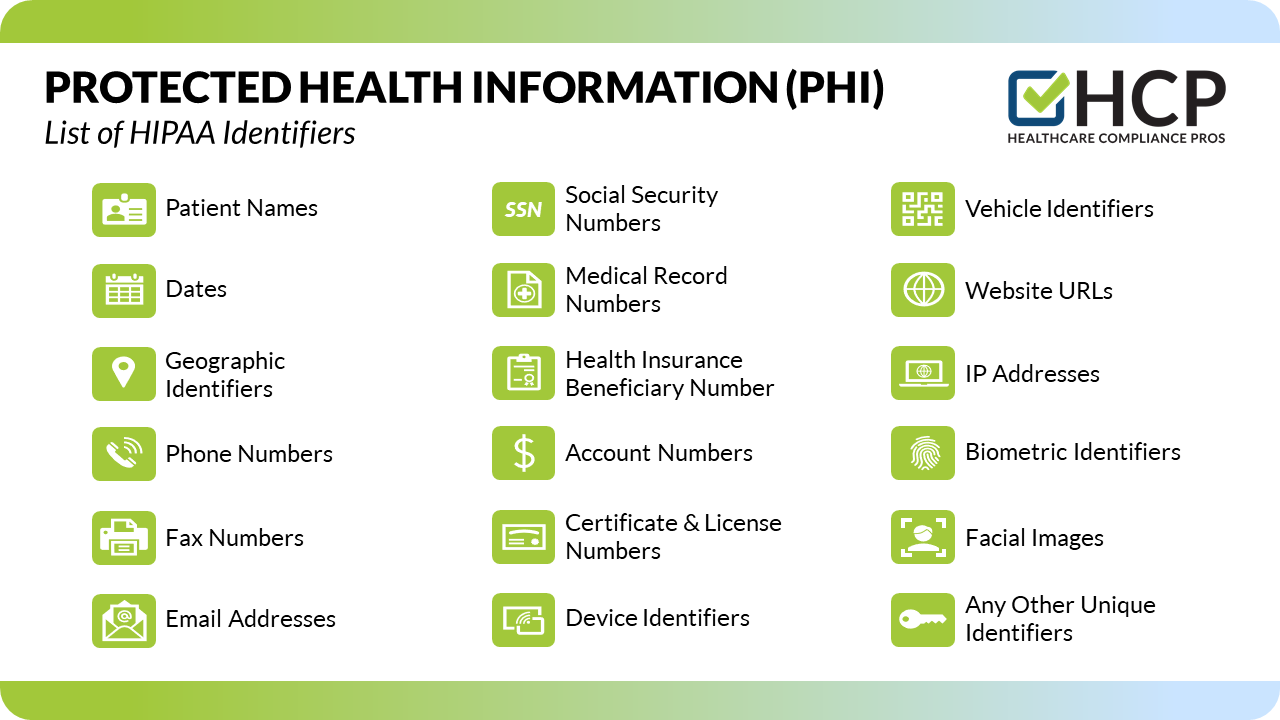
HIPAA identifies 18 specific identifiers that, if associated with health information, render the data PHI. These include names, addresses, dates of birth, Social Security numbers, medical record numbers, health plan beneficiary numbers, account numbers, certificate/license numbers, vehicle identifiers, device identifiers, URLs, IP addresses, biometric identifiers, and full face photographic images.
Exclusions from PHI Protection
While the scope of PHI is broad, certain types of data are specifically excluded from these protections. These exclusions generally fall into several categories: de-identified data, employment records, and educational records.
De-identified data, as the name suggests, is health information that has been stripped of all 18 identifiers, rendering it impossible to trace back to a specific individual. This type of data is often used for research and public health purposes.
Data is considered de-identified only if a qualified statistician has determined that the risk of re-identification is very small, or if the covered entity has obtained formal certification of de-identification. Simply removing a few obvious identifiers is insufficient.
Employment records held by covered entities in their role as *employers* are typically not considered PHI. For example, if a hospital maintains employee health records for occupational health and safety purposes, this information is generally governed by employment laws, not HIPAA.
Educational records covered under the Family Educational Rights and Privacy Act (FERPA) are also generally excluded. This means that health information contained within a student's educational record at a school or university is subject to FERPA's protections, rather than HIPAA's.
The Nuances and Grey Areas
Determining whether specific information qualifies as PHI is not always straightforward. There exist several nuanced scenarios and grey areas that require careful consideration.
For example, information that is publicly available, such as a patient's name listed in a hospital directory, may not be considered PHI in all contexts. However, its use in conjunction with other health information could trigger PHI status.
Metadata associated with electronic health records, such as audit trails documenting who accessed a patient's chart, can also raise complex questions. Whether such metadata is considered PHI depends on its potential to identify the individual to whom the record pertains.
"The definition of PHI hinges on the ability to identify an individual," explains Dr. Emily Carter, a privacy law expert. "The key is to assess whether the information, alone or in combination with other reasonably available data, can be used to pinpoint a specific person."
Implications of Exclusions
The exclusions from PHI protection have significant implications for both patients and healthcare providers. Understanding these implications is crucial for maintaining ethical and legal compliance.
While de-identified data is intended to be used for research and public health, concerns remain about the potential for re-identification. Advances in data analytics and computing power make it increasingly challenging to guarantee complete anonymity.
The exclusion of employment records highlights the importance of separate data protection policies for employees. Covered entities must ensure that employee health information is handled appropriately under relevant employment laws.
The FERPA exclusion underscores the need for educational institutions to understand their own data protection obligations. Students' health information contained in educational records warrants careful handling under FERPA's rules.
Looking Ahead
The landscape of patient information protection is constantly evolving. Technological advancements, changing regulatory requirements, and increasing public awareness of privacy issues are driving the need for ongoing clarification and refinement of these rules.
Healthcare providers and policymakers must prioritize patient education and transparency. Patients should be informed about what data is considered PHI and how it is protected.
Continued research and development are needed to enhance de-identification techniques and minimize the risk of re-identification. Maintaining public trust in healthcare requires a commitment to protecting patient privacy while enabling the beneficial use of health data for research and innovation.
Ultimately, a balanced approach is necessary. Safeguarding sensitive information while facilitating legitimate uses of health data is a challenge that demands ongoing dialogue and collaboration among all stakeholders.







![Patient Information Includes All Of The Following Except The [ANSWERED] Innate immunity includes all of the following EXCEPT O - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20220611194806688881-4527172.jpg?h=512)