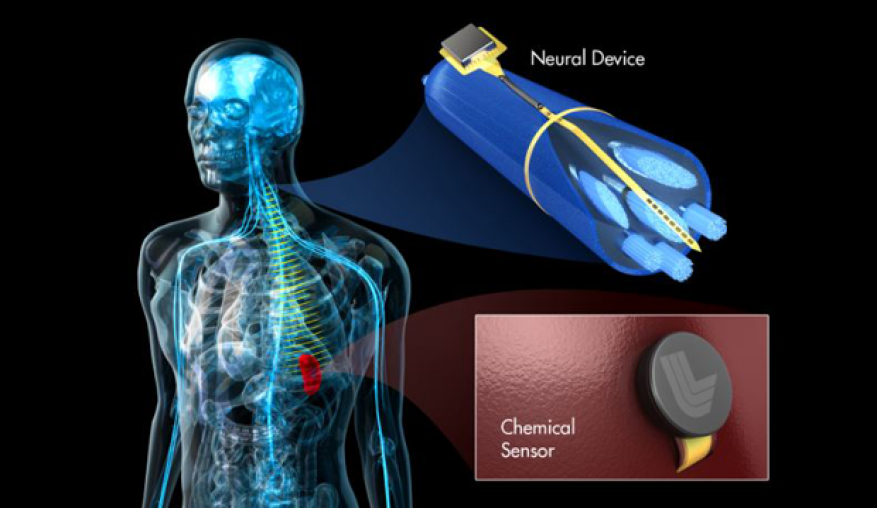
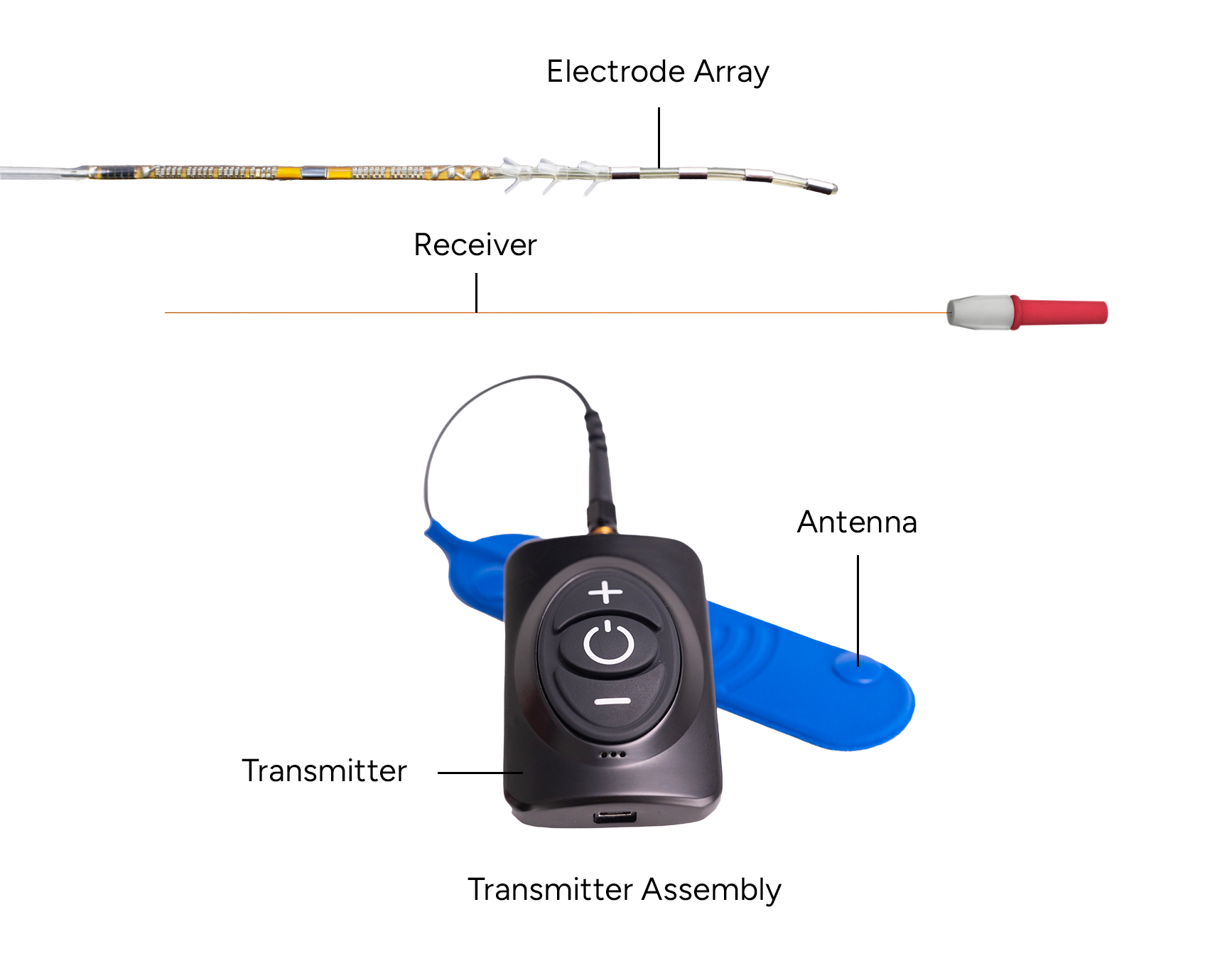
Peripheral Nerve Stimulators Medical Devices Pipeline Product Analysis

The peripheral nerve stimulation (PNS) devices market is poised for significant growth, driven by increasing demand for non-opioid pain management solutions. A surge in research and development activities is fueling a dynamic pipeline of innovative PNS devices, offering hope for patients suffering from chronic pain.
This report analyzes the current PNS medical device pipeline, identifying key players, product development stages, and emerging technologies. The analysis reveals a competitive landscape with promising advancements aimed at improving patient outcomes and expanding treatment options.
Market Overview and Growth Drivers
The global peripheral nerve stimulation market is experiencing robust growth, projected to reach multi-billion dollar valuations within the next decade.
Several factors contribute to this growth, including the rising prevalence of chronic pain conditions, an aging population, and growing concerns about opioid addiction. A greater emphasis on minimally invasive procedures and patient preference for non-pharmacological interventions are also driving market expansion.
The demand for effective pain management solutions is creating a fertile ground for innovation in the PNS device sector.
Key Players and Product Pipeline
Several companies are actively involved in the development and commercialization of PNS devices. These include established medical device giants and smaller, innovative startups. Medtronic, Abbott, and Boston Scientific are major players with established portfolios.
Nevro Corp is also making significant strides with its Senza system. Emerging companies like SPR Therapeutics and Stimwave are introducing novel technologies to the market.
Product development ranges from early-stage research to late-stage clinical trials and commercial launches. Key areas of innovation include: wireless PNS devices, miniaturized implants, closed-loop stimulation systems, and targeted nerve stimulation technologies.
Wireless PNS Devices
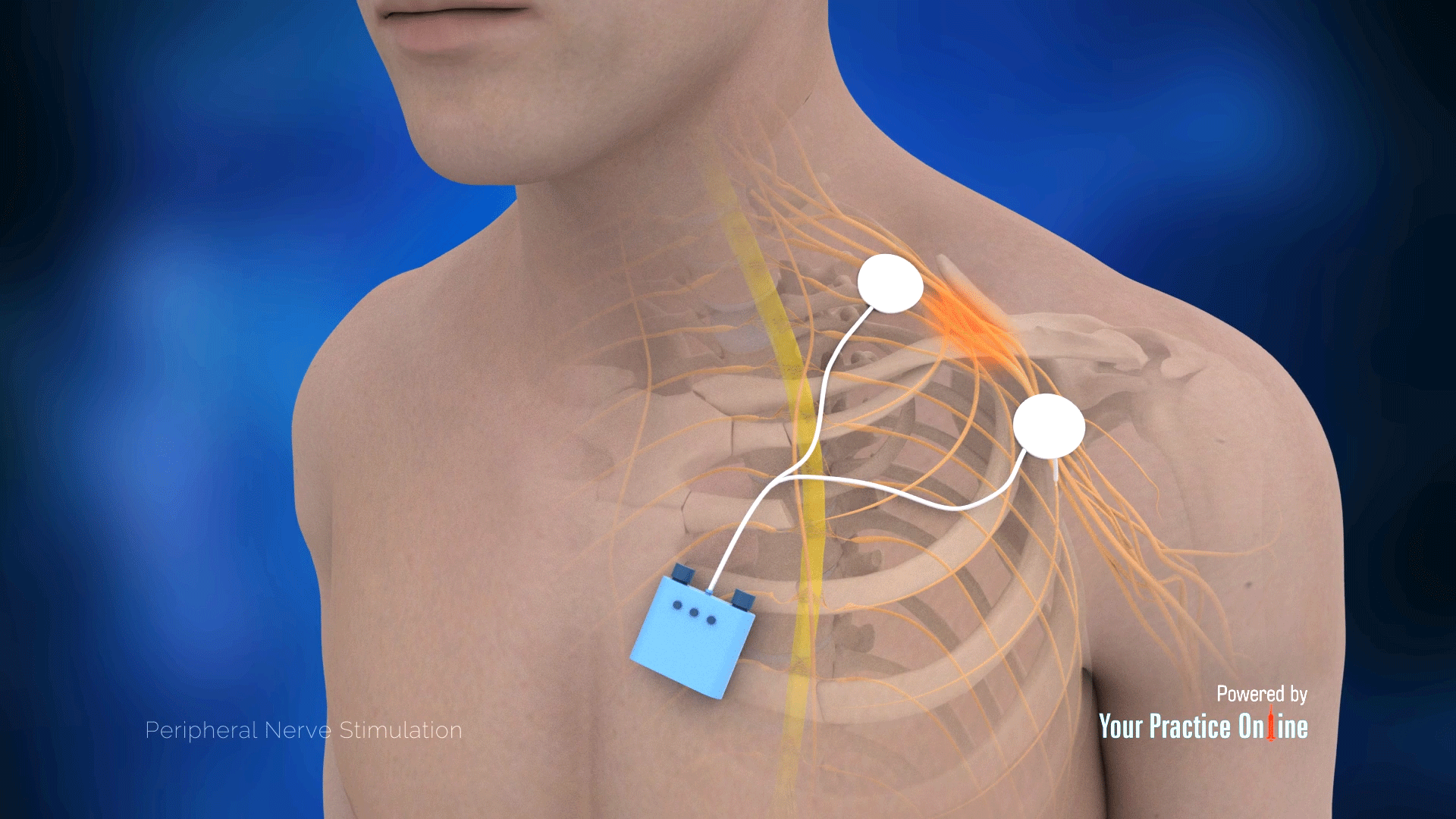
Wireless PNS devices eliminate the need for external pulse generators, improving patient comfort and convenience. These devices often utilize percutaneous placement, making them minimally invasive.
SPR Therapeutics' SPRINT PNS System is an example of a wireless system that has demonstrated promising results in treating chronic pain.
Several other companies are actively developing wireless PNS technologies, contributing to a competitive landscape.
Miniaturized Implants
Miniaturization is another key trend in the PNS device pipeline. Smaller implants offer improved patient comfort and reduced risk of complications.
These devices are designed for targeted nerve stimulation, delivering precise therapy to the affected area. Advances in microelectronics and biomaterials are enabling the development of increasingly smaller and more sophisticated implants.
Closed-Loop Stimulation Systems
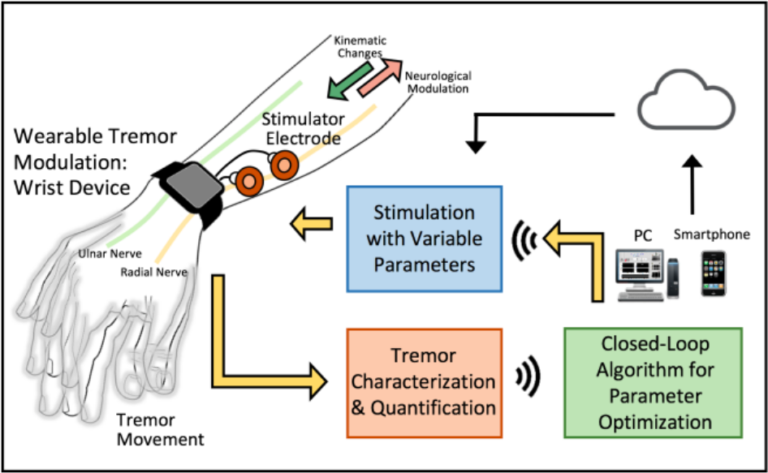
Closed-loop stimulation systems automatically adjust stimulation parameters based on real-time feedback from the patient's nervous system. This personalized approach to pain management has the potential to improve treatment efficacy and reduce side effects.
Research is ongoing to develop sophisticated algorithms and sensors for closed-loop PNS systems. These systems represent a significant advancement over traditional open-loop systems.
Targeted Nerve Stimulation Technologies
Targeted nerve stimulation technologies aim to deliver precise stimulation to specific nerves or nerve fibers. This approach minimizes off-target effects and maximizes therapeutic benefits.
Advanced imaging techniques and electrode designs are enabling more precise targeting of peripheral nerves. This precision is particularly important for treating complex pain conditions.
Clinical Trial Landscape
Numerous clinical trials are underway to evaluate the safety and efficacy of novel PNS devices. These trials are crucial for obtaining regulatory approvals and demonstrating the clinical value of new technologies.
Clinical trial endpoints typically include pain reduction, functional improvement, and quality of life assessment. Rigorous clinical evidence is essential for driving adoption of PNS devices among physicians and patients.
Regulatory Considerations
PNS devices are subject to strict regulatory oversight by agencies such as the FDA in the United States and the EMA in Europe. Regulatory pathways vary depending on the device's classification and intended use.
Companies must demonstrate the safety and efficacy of their devices through preclinical testing and clinical trials. Obtaining regulatory approvals is a critical milestone for commercializing new PNS technologies.
Challenges and Opportunities
Despite the promising outlook, the PNS device market faces several challenges. These include: high device costs, limited insurance coverage, and the need for specialized training for physicians.
Opportunities exist to address these challenges through: development of more cost-effective devices, advocacy for broader insurance coverage, and expansion of physician training programs. Continued innovation and clinical research are also essential for driving market growth.
The adoption of PNS devices is dependent on demonstrating their cost-effectiveness and clinical superiority compared to traditional pain management methods.
Conclusion and Next Steps
The PNS medical device pipeline is robust and dynamic, with numerous promising technologies under development. The increasing demand for non-opioid pain management solutions is driving innovation and investment in this sector.
Ongoing clinical trials and regulatory approvals will be critical for shaping the future of the PNS market. Continued advancements in wireless technology, miniaturization, and closed-loop stimulation are expected to further enhance the efficacy and patient acceptance of PNS devices.
Stakeholders should monitor clinical trial results, regulatory decisions, and emerging technologies to stay abreast of developments in the PNS market. Collaboration between industry, researchers, and clinicians is essential for accelerating innovation and improving patient outcomes.