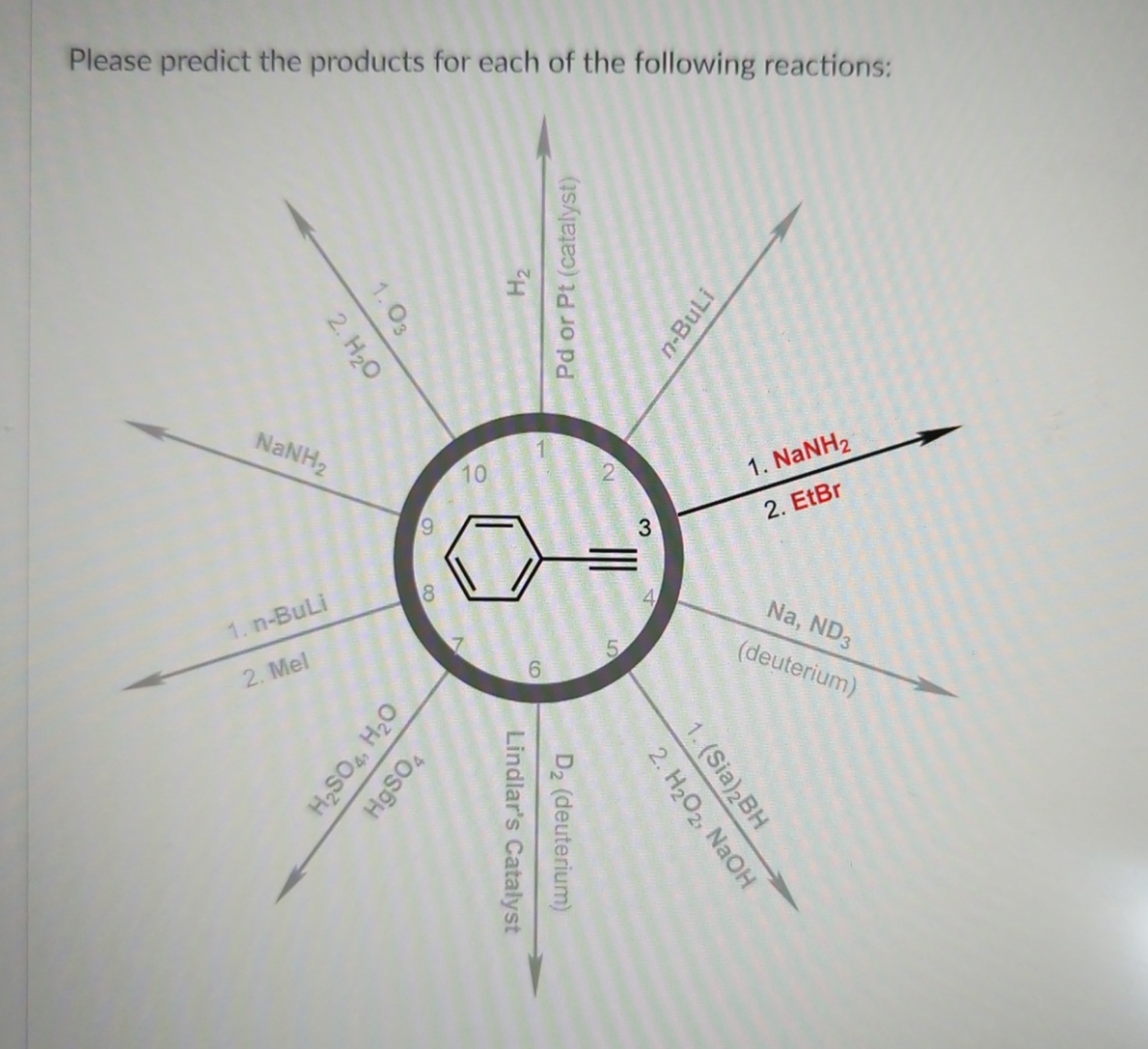
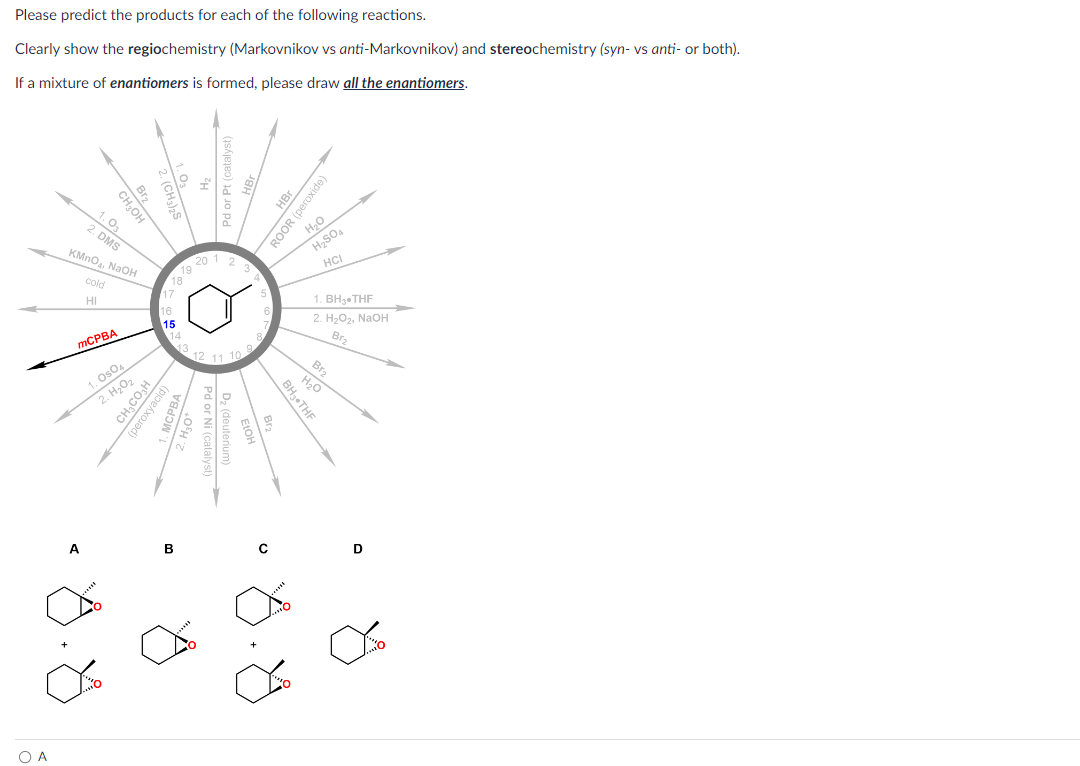
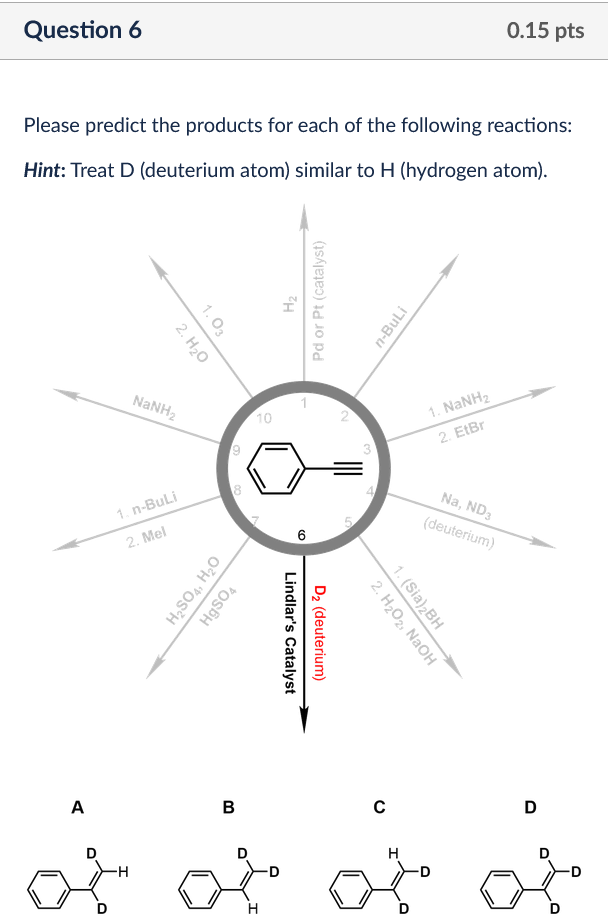
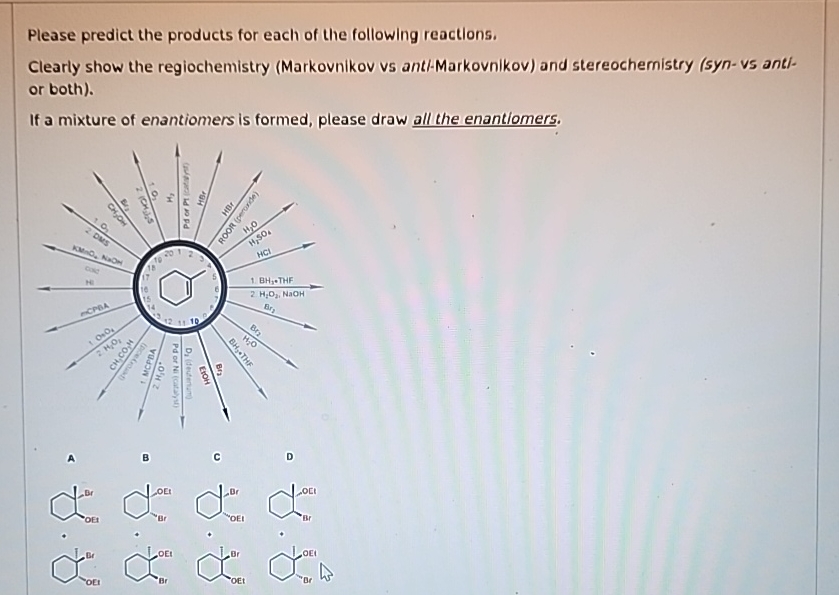
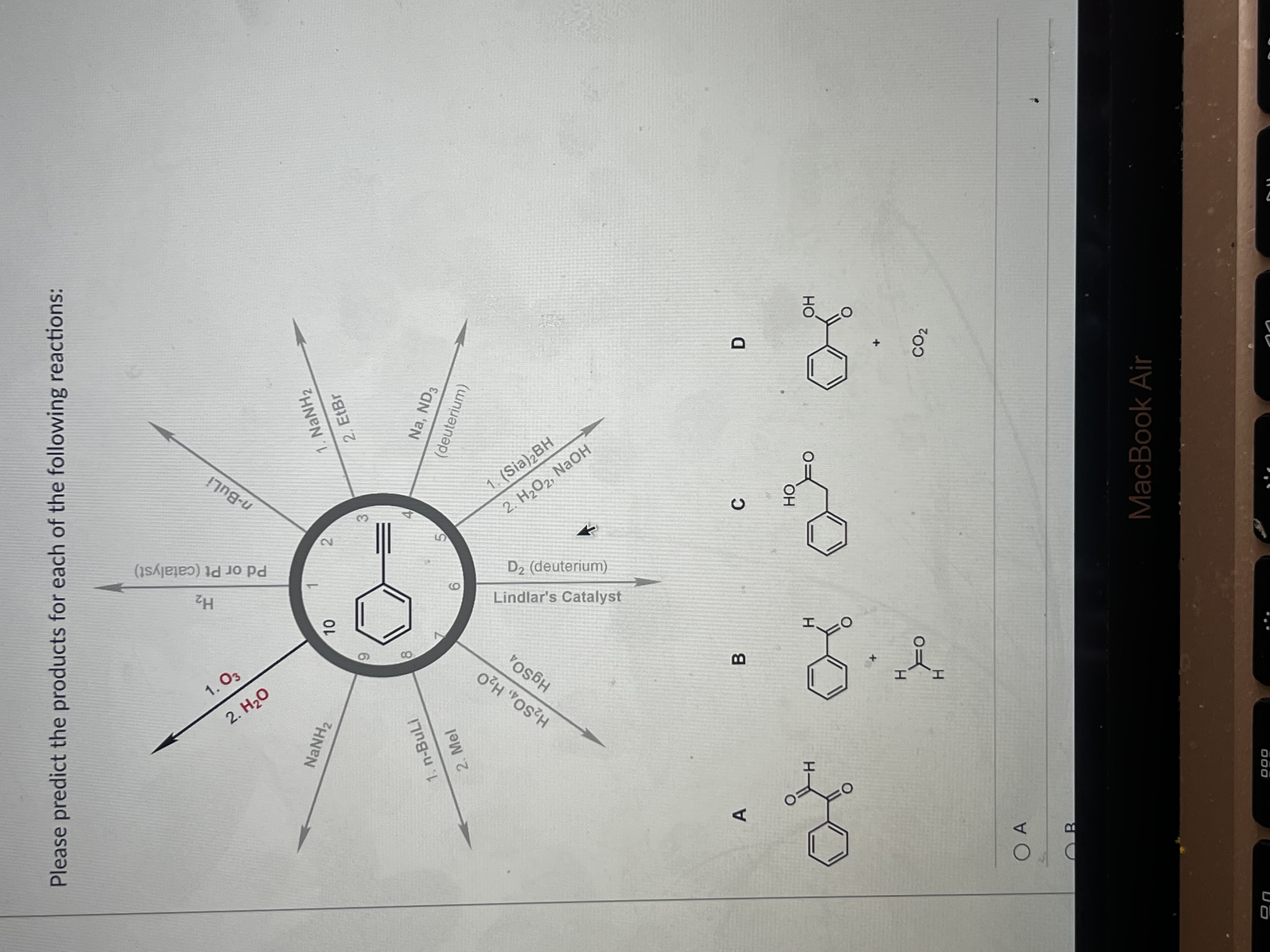
Please Predict The Products For Each Of The Following Reactions:
Predicting the outcome of chemical reactions is a cornerstone of chemistry, vital for fields ranging from drug discovery to materials science. While seemingly confined to the laboratory, the ability to accurately forecast reaction products has profound implications for everyday life, influencing the development of new technologies and the optimization of industrial processes.
Chemical reactions involve the rearrangement of atoms and molecules, leading to the formation of new substances. Precisely predicting the products of these reactions requires a deep understanding of chemical principles, including stoichiometry, thermodynamics, and kinetics.
Below are hypothetical scenarios outlining chemical reactions, along with likely predicted products based on established chemical knowledge.
Reaction 1: Neutralization Reaction
The reaction involves a strong acid, hydrochloric acid (HCl), with a strong base, sodium hydroxide (NaOH).
The balanced chemical equation would be: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l). The predicted products are sodium chloride (NaCl), common table salt, dissolved in water (H2O).
This is a classic neutralization reaction, where the acid and base react to form a salt and water. Neutralization reactions are highly exothermic.
Reaction 2: Single Displacement Reaction
Solid zinc (Zn) is added to a solution of copper(II) sulfate (CuSO4).
The predicted products are solid copper (Cu) and zinc sulfate (ZnSO4) in solution: Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq). Zinc is more reactive than copper, so it displaces copper from the sulfate solution.
This reaction demonstrates the concept of the activity series of metals, which ranks metals based on their relative ease of oxidation. This forms the basis of batteries.
Reaction 3: Combustion Reaction
Methane gas (CH4) is burned in the presence of excess oxygen (O2).
The predicted products are carbon dioxide (CO2) and water (H2O): CH4(g) + 2O2(g) → CO2(g) + 2H2O(g). Combustion reactions are exothermic, releasing heat and light.
This is a fundamental reaction for energy production, powering vehicles and generating electricity. Incomplete combustion can produce carbon monoxide, a dangerous gas.
Reaction 4: Precipitation Reaction
Aqueous solutions of silver nitrate (AgNO3) and sodium chloride (NaCl) are mixed.
The predicted products are solid silver chloride (AgCl) and aqueous sodium nitrate (NaNO3): AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq). Silver chloride is insoluble in water and forms a precipitate.
The formation of a precipitate is a key indicator of a chemical reaction. Understanding solubility rules is crucial for predicting precipitation reactions.
Reaction 5: Acid-Base Reaction with Gas Evolution
Solid calcium carbonate (CaCO3) reacts with hydrochloric acid (HCl).
The predicted products are calcium chloride (CaCl2), water (H2O), and carbon dioxide gas (CO2): CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g). The evolution of gas is a characteristic feature of this type of reaction.
This reaction is responsible for the dissolving of limestone by acid rain. The escaping carbon dioxide contributes to greenhouse gasses in the atmosphere.
Reaction 6: Synthesis Reaction
Hydrogen gas (H2) reacts with nitrogen gas (N2) under high pressure and temperature with a catalyst.
The predicted product is ammonia gas (NH3): N2(g) + 3H2(g) → 2NH3(g). This is the Haber-Bosch process, an important industrial reaction.
The Haber-Bosch process is vital for producing fertilizers and has dramatically increased agricultural yields. This process has a significant impact on global food production.
Reaction 7: Decomposition Reaction
Hydrogen peroxide (H2O2) slowly decomposes at room temperature.
The predicted products are water (H2O) and oxygen gas (O2): 2H2O2(aq) → 2H2O(l) + O2(g). Catalysts like manganese dioxide can speed up the decomposition.
This reaction is used in disinfectants and bleaching agents. Decomposition reactions are the opposite of synthesis reactions.
The ability to predict the products of chemical reactions is not simply an academic exercise. It is a fundamental skill that drives innovation in a wide range of industries and contributes to our understanding of the world around us.
By mastering the principles of chemical reactions, scientists and engineers can develop new materials, design more efficient processes, and create solutions to some of the world's most pressing challenges. Continued research and development in this area will undoubtedly lead to even greater advancements in the future.