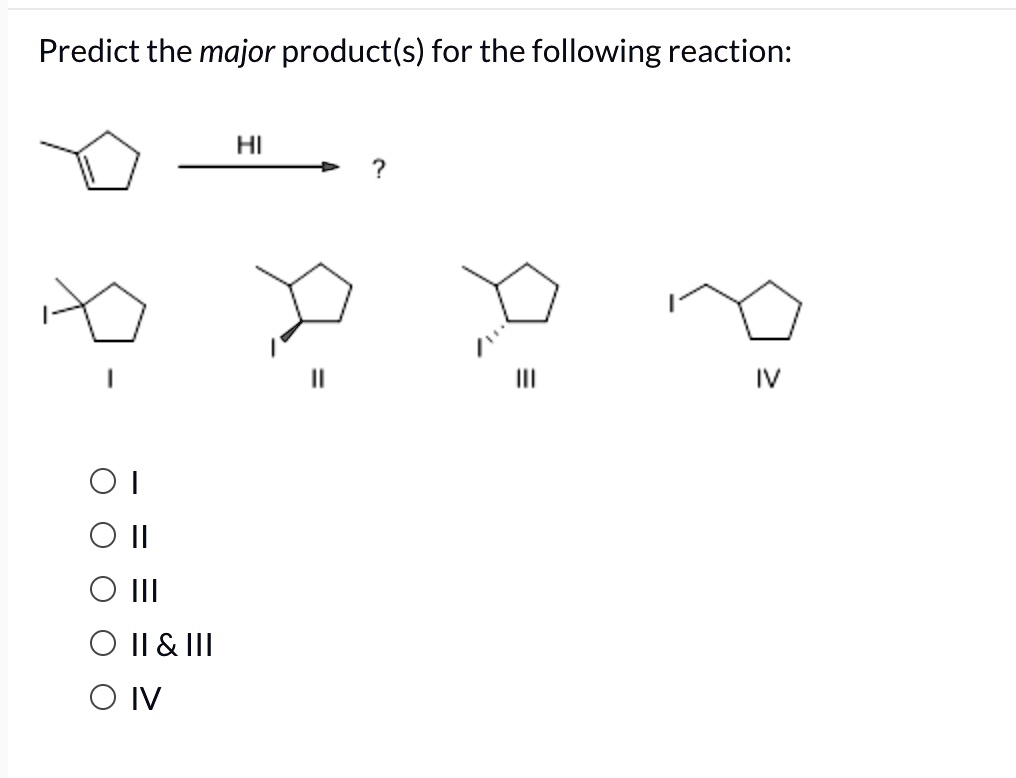
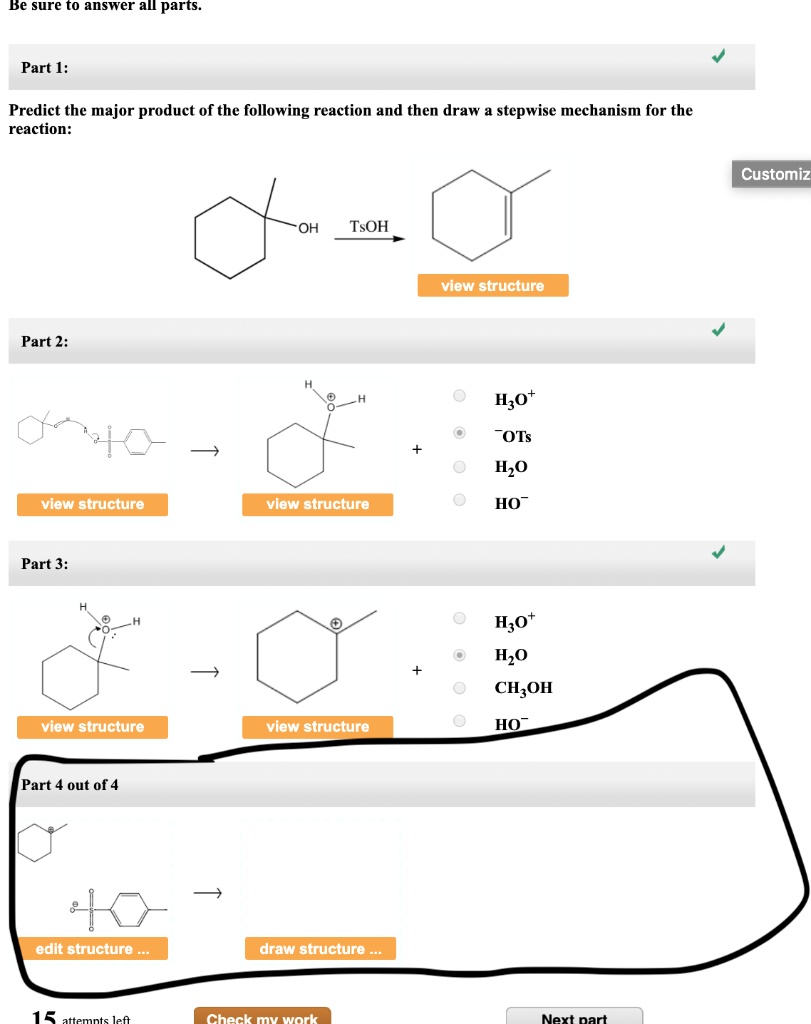
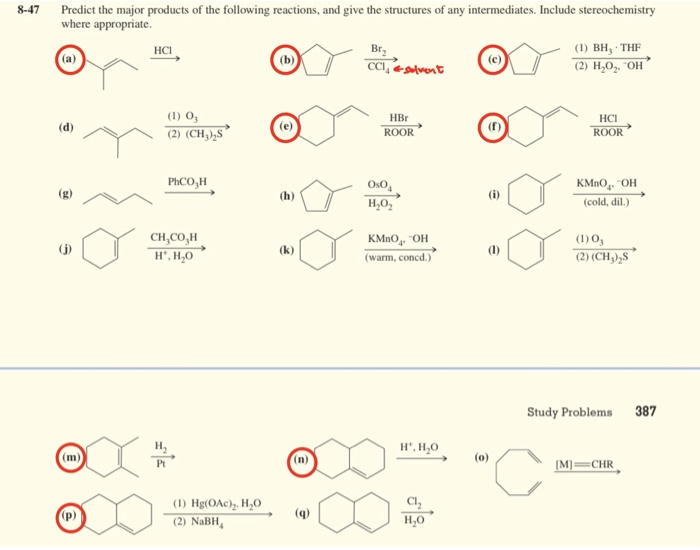
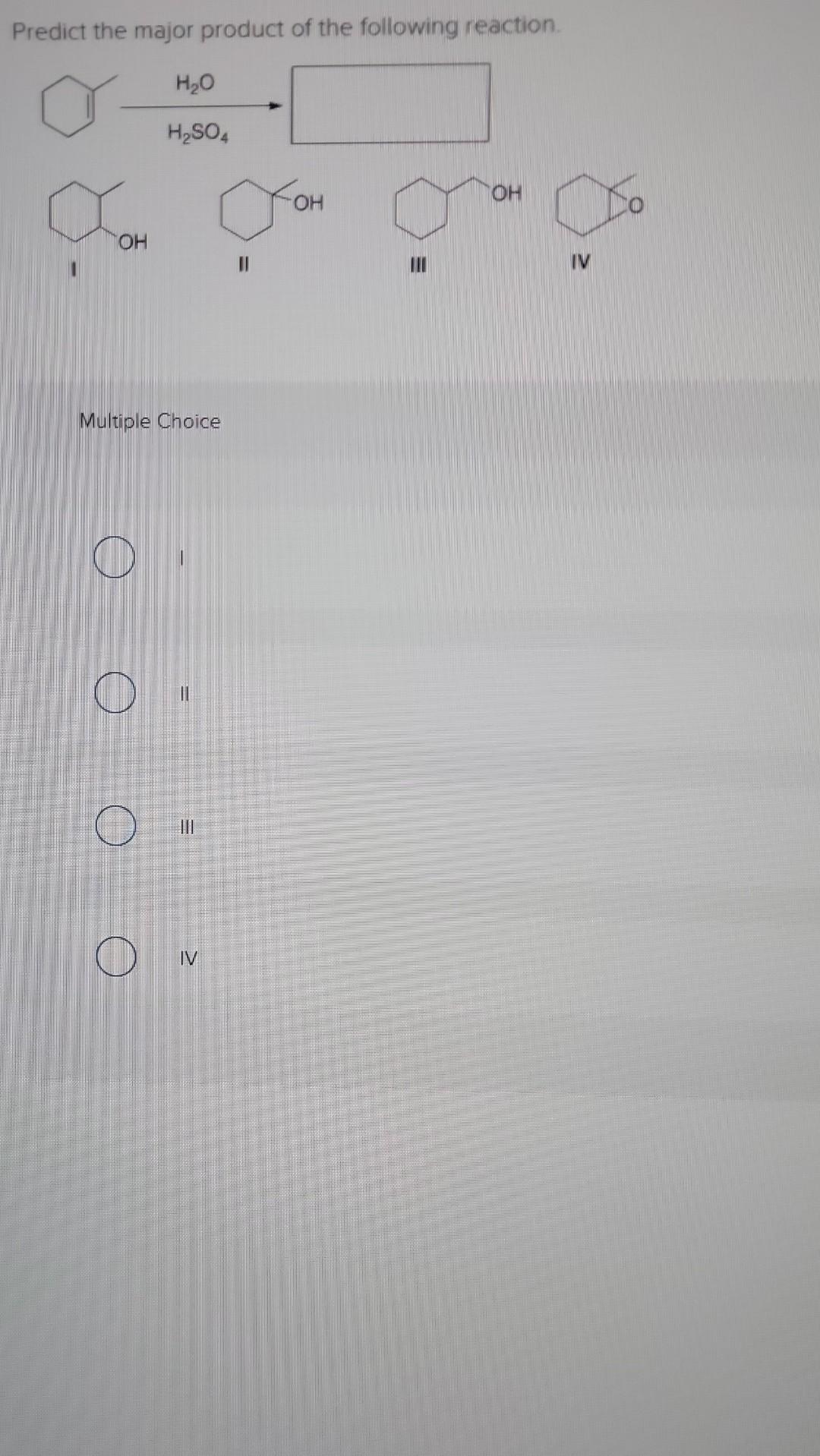
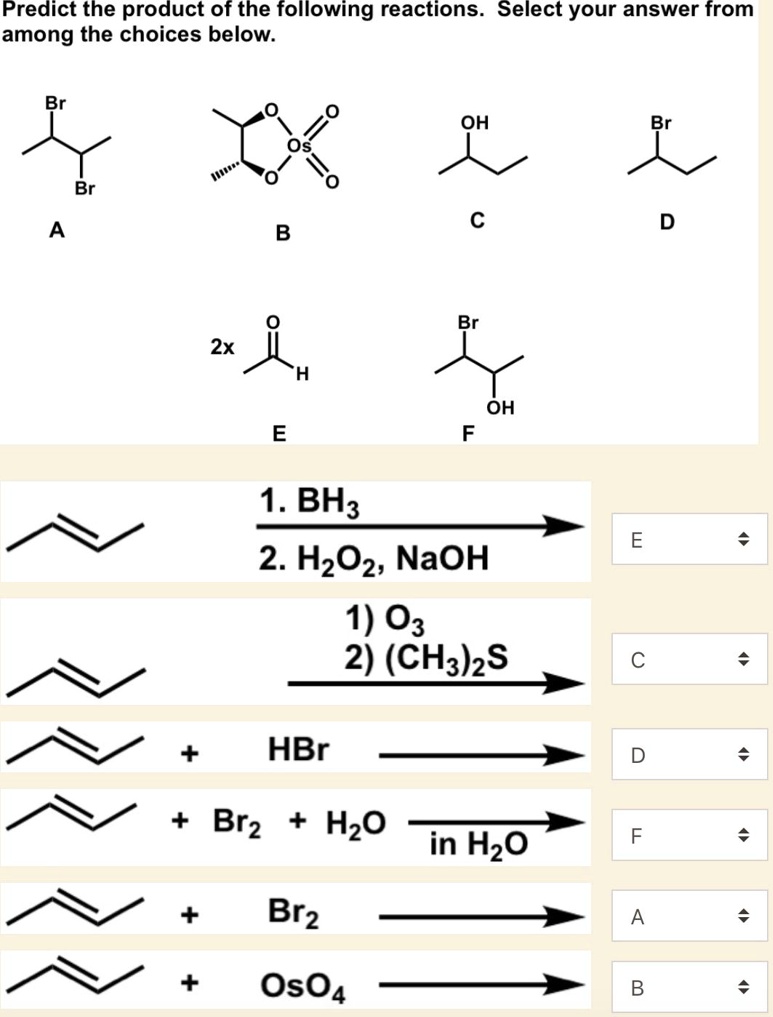
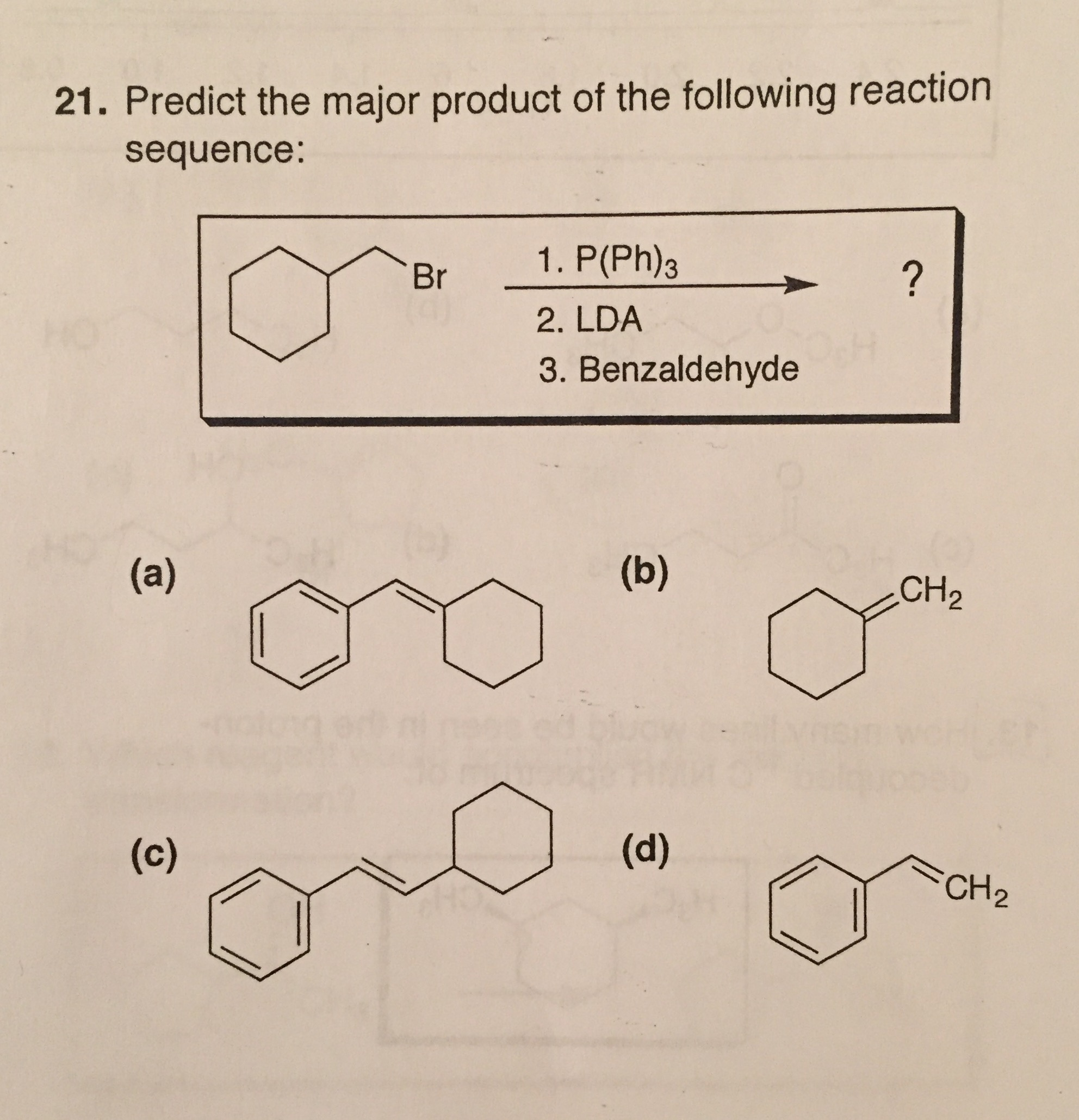
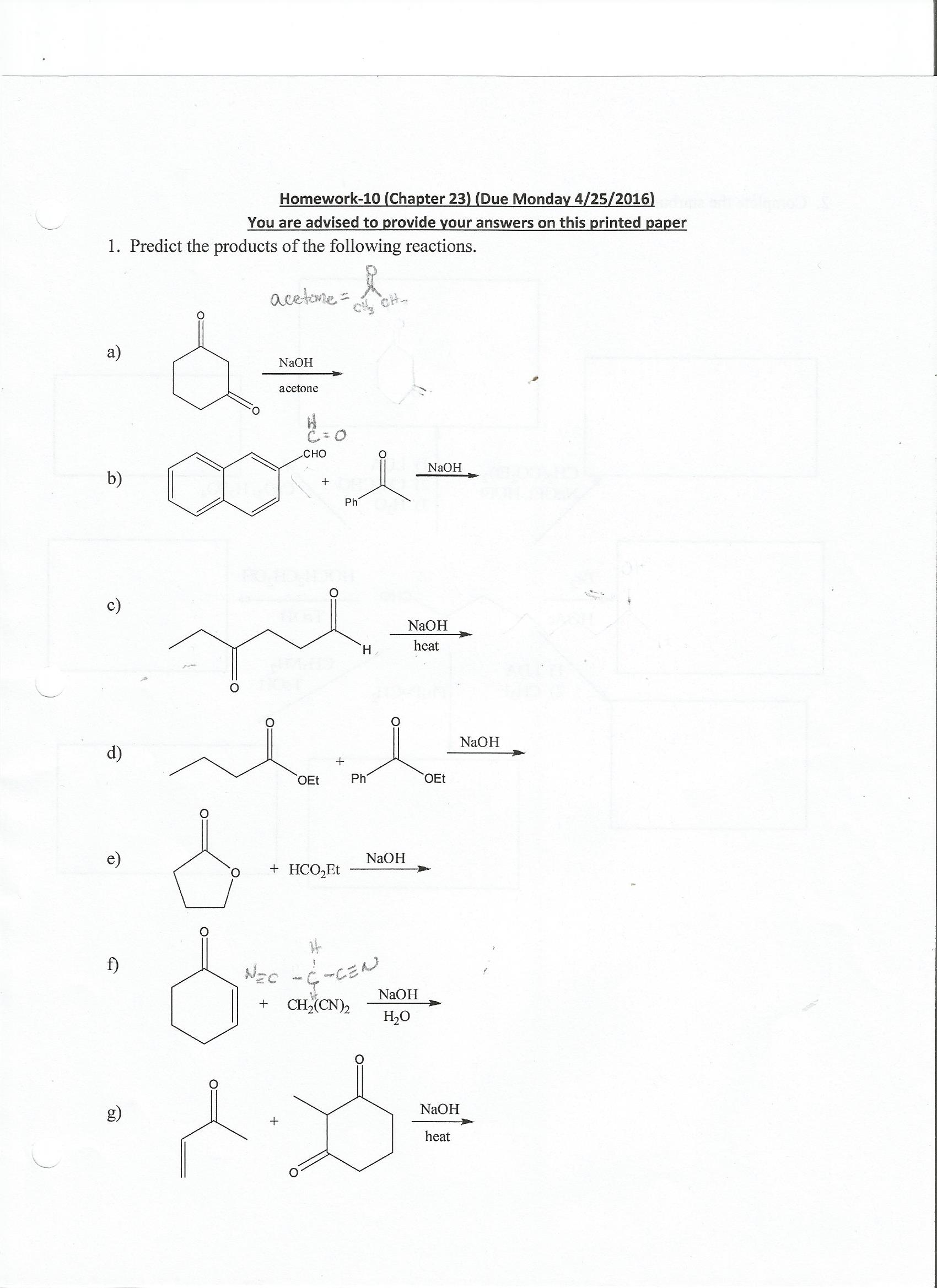
Predict The Product Of The Following Reactions
Imagine stepping into a bustling chemistry lab, the air thick with the subtle aroma of reagents, glassware gleaming under the soft fluorescent lights. Beakers bubble gently on hot plates, while students huddle around whiteboards, their faces illuminated by a mixture of concentration and excitement. They're not just mixing chemicals; they're unraveling the mysteries of molecular interactions, predicting the invisible dance of atoms.
At the heart of this endeavor lies a fundamental skill: predicting the products of chemical reactions. This capability is not merely an academic exercise but a cornerstone of scientific advancement, impacting fields from medicine to materials science.
The Foundation: Understanding Chemical Reactions
To understand product prediction, it’s essential to grasp the basic principles of chemical reactions. A chemical reaction involves the rearrangement of atoms and molecules, breaking existing bonds and forming new ones.
This process is governed by fundamental laws of chemistry, including the conservation of mass and energy. Mastering these basics lays the groundwork for understanding the forces that drive reactions.
Balancing Equations: Ensuring Conservation
A crucial aspect of predicting products is ensuring that chemical equations are properly balanced. Balancing ensures that the number of atoms of each element is the same on both sides of the equation, adhering to the law of conservation of mass.
This involves carefully adjusting coefficients in front of chemical formulas to equalize the number of each type of atom.
Types of Chemical Reactions: A Categorical Approach
Chemical reactions can be classified into several broad categories, each with characteristic patterns. Understanding these categories simplifies the prediction process.
Synthesis reactions involve combining two or more reactants to form a single product. Decomposition reactions are the opposite, where a single reactant breaks down into two or more products.
Single displacement reactions involve one element replacing another in a compound. Double displacement reactions involve the exchange of ions between two compounds, often resulting in the formation of a precipitate, a gas, or water.
Combustion reactions involve the rapid reaction between a substance with an oxidant, usually oxygen, to produce heat and light. These reactions often generate carbon dioxide and water as products.
Tools of the Trade: Reaction Mechanisms and Catalysts
Delving deeper, reaction mechanisms offer a step-by-step description of how a reaction occurs. These mechanisms illustrate the movement of electrons and the formation of intermediates.
Understanding reaction mechanisms provides insight into the reaction pathway, enabling more accurate predictions of product formation.
Catalysts play a crucial role in many chemical reactions by accelerating the rate of reaction without being consumed themselves. They achieve this by lowering the activation energy required for the reaction to proceed.
Identifying the presence and role of catalysts is vital for accurately predicting reaction outcomes.
Predicting Products: A Step-by-Step Approach
The process of predicting products typically involves a structured approach. First, it’s necessary to identify the reactants and their chemical properties.
Next, one must determine the type of reaction that is likely to occur based on the reactants involved. Consider the likely rearrangement of atoms and molecules, balancing charges and satisfying valency rules.
Finally, double-check that the equation is balanced, ensuring the conservation of mass.
Example 1: Synthesis of Water
Consider a simple example: the synthesis of water from hydrogen and oxygen. The reactants are hydrogen gas (H2) and oxygen gas (O2).
This is a synthesis reaction, where the two gases combine to form a single product: water (H2O). The balanced equation is 2H2 + O2 → 2H2O.
Example 2: Neutralization Reaction
Another example is the reaction between a strong acid, such as hydrochloric acid (HCl), and a strong base, such as sodium hydroxide (NaOH). This is a neutralization reaction, a type of double displacement.
The products are salt (NaCl) and water (H2O). The balanced equation is HCl + NaOH → NaCl + H2O.
Applications in the Real World
The ability to predict the products of chemical reactions has profound implications across various fields. In the pharmaceutical industry, it is crucial for designing and synthesizing new drugs.
In materials science, it enables the development of novel materials with specific properties. In environmental science, it aids in understanding and mitigating pollution.
Pharmaceuticals: Designing New Medicines
Predicting reaction outcomes is essential in the development and manufacturing of pharmaceuticals. Chemists need to accurately predict the products and yields of reactions to synthesize drug molecules efficiently.
This ensures that the desired drug is produced with minimal waste and maximum purity, ultimately benefiting patients.
Materials Science: Creating Innovative Materials
In materials science, product prediction is critical for designing new materials with tailored properties. By understanding how different elements and compounds react, scientists can create materials with specific strength, conductivity, or other desired characteristics.
This ability leads to innovations in electronics, construction, and other industries.
Environmental Science: Protecting the Planet
Environmental scientists use the knowledge of chemical reactions to understand and address pollution problems. By predicting the products of reactions involving pollutants, they can develop strategies for remediation and prevention.
This might involve designing catalysts that break down pollutants into less harmful substances or predicting the fate of pollutants in the environment.
Challenges and Future Directions
Despite the advances in computational chemistry, accurately predicting reaction products remains a challenge, especially for complex organic reactions. Factors such as solvent effects, temperature, and pressure can significantly influence reaction outcomes.
Current research is focused on developing more sophisticated computational models and experimental techniques to improve predictive accuracy. Quantum mechanical calculations and machine learning algorithms are showing promise in this area.
Looking ahead, the integration of artificial intelligence and machine learning will revolutionize our ability to predict reaction outcomes. These technologies can analyze vast amounts of chemical data to identify patterns and predict reaction pathways with greater accuracy.
This will accelerate the pace of discovery and innovation across many fields.
From the simplest synthesis to the most complex organic transformation, the ability to predict the products of chemical reactions empowers us to understand and manipulate the molecular world. It is a skill that fuels scientific progress, drives innovation, and ultimately improves our lives.
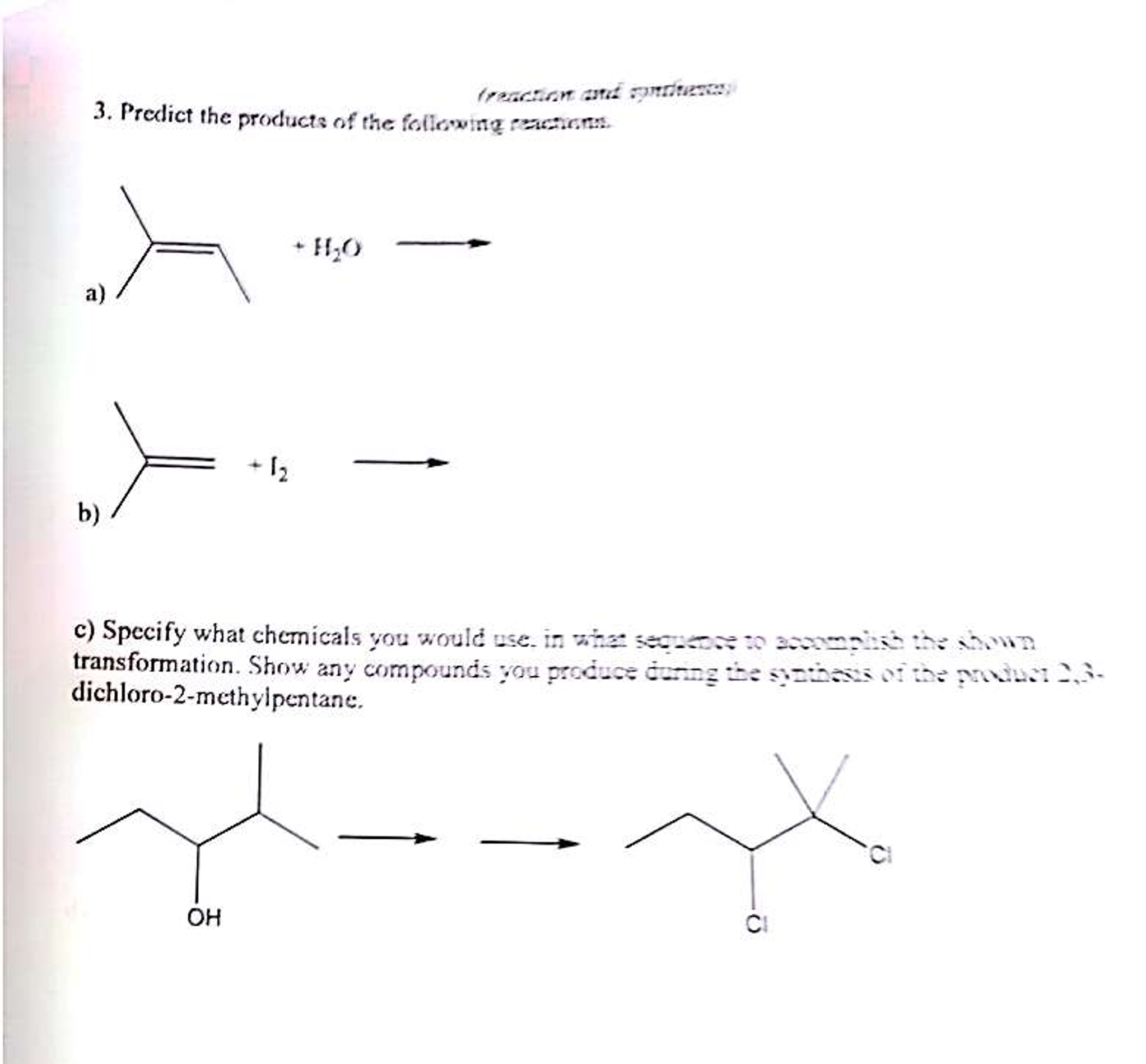




![Predict The Product Of The Following Reactions Solved [2] Predict the product of the following reaction. 1. | Chegg.com](https://media.cheggcdn.com/media/86c/86c041b2-f348-475b-8518-9568911f193b/image.png)