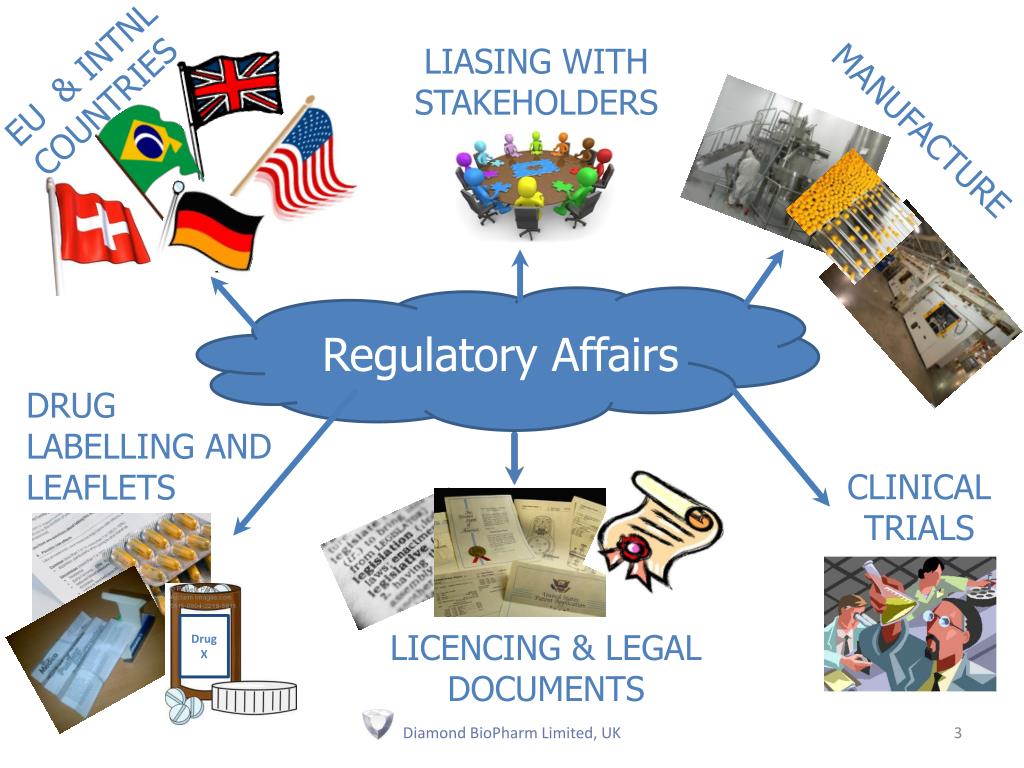
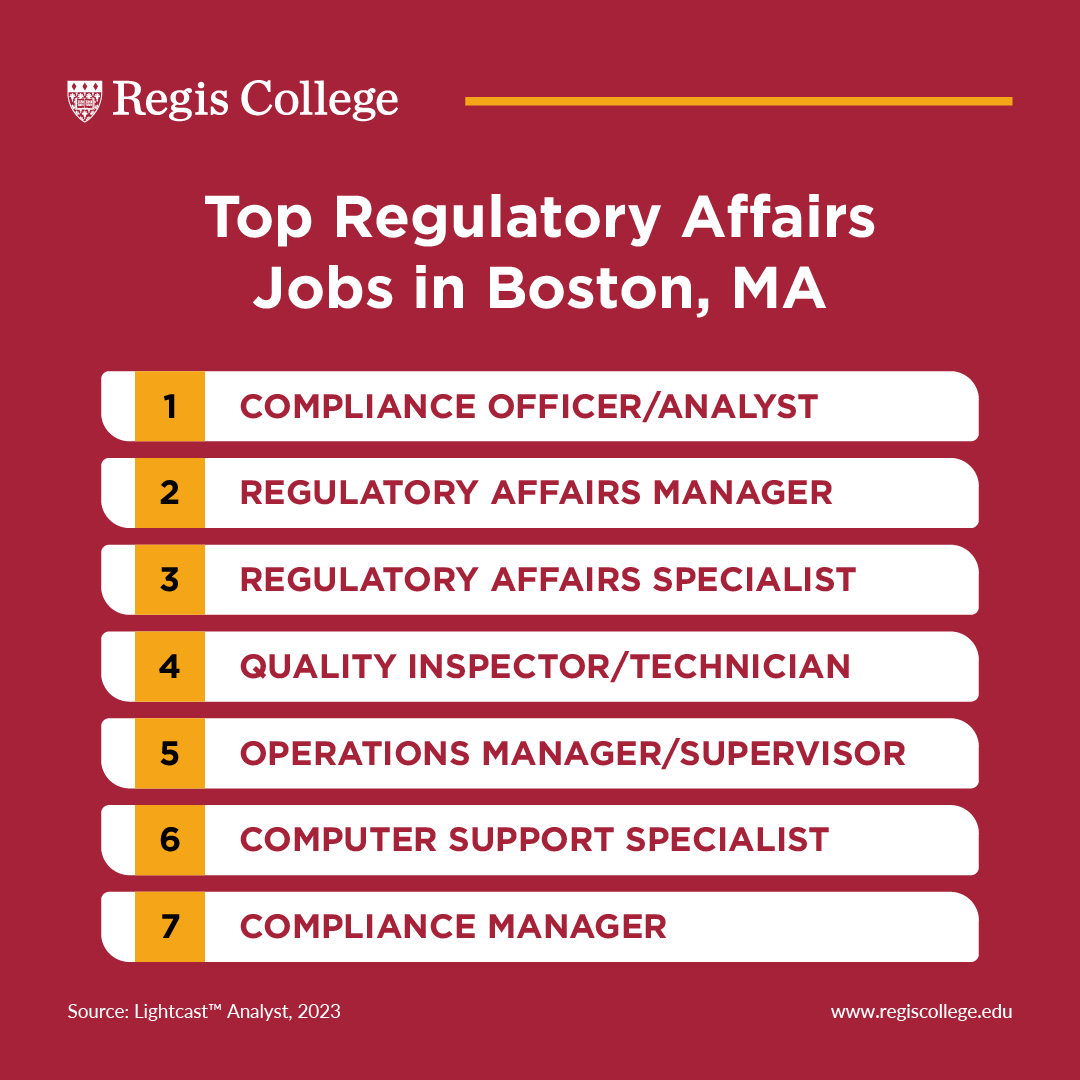
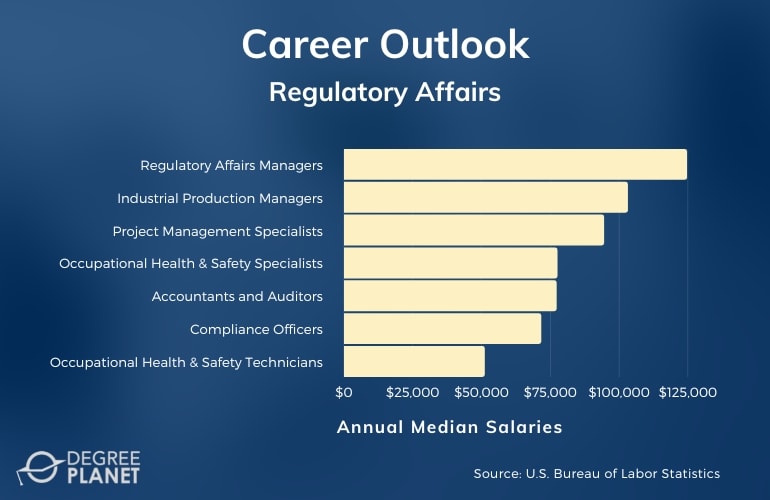
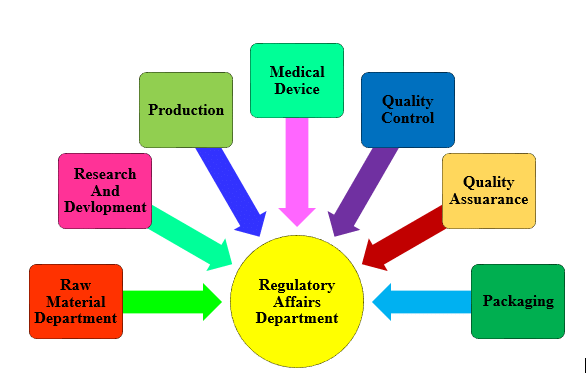
Regulatory Affairs Universities In Usa
The pharmaceutical and medical device industries, juggernauts of innovation and public health, are facing a critical challenge: a growing demand for skilled regulatory affairs professionals. This surge, fueled by increasingly complex regulatory landscapes and accelerated drug development timelines, is prompting a re-evaluation of how these vital specialists are trained. Universities across the United States are stepping up to meet this need, but questions remain about the standardization and accessibility of these programs.
The need for adequately trained professionals is underscored by the projected growth in the regulatory affairs job market. This demand necessitates a robust educational infrastructure capable of producing knowledgeable and adaptable graduates. The core issue revolves around ensuring these university programs adequately equip students with the skills to navigate the intricate web of regulations governing the development, approval, and marketing of life-saving therapies.
The Landscape of Regulatory Affairs Programs
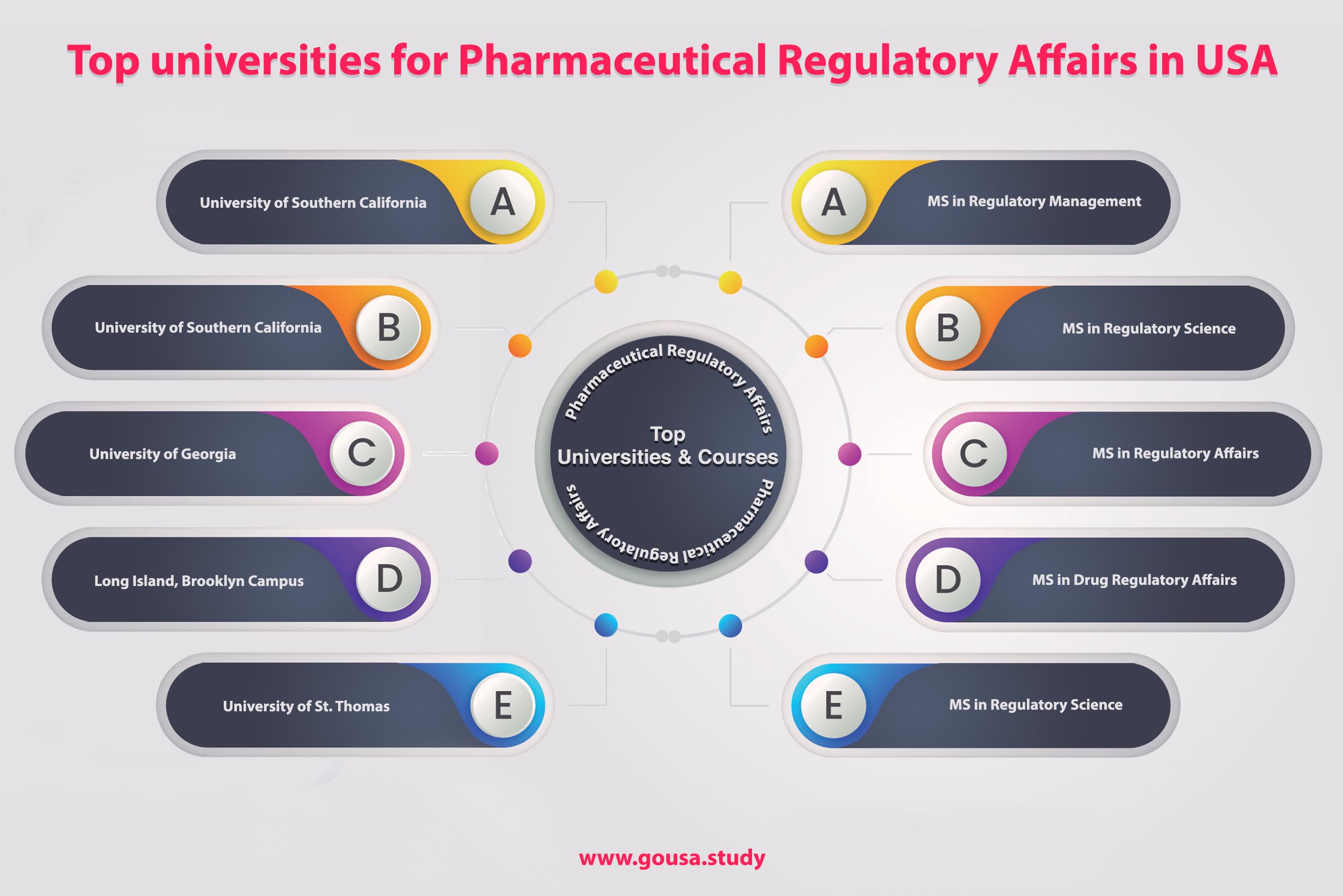
Several universities across the U.S. offer specialized programs in regulatory affairs, typically at the master's level. These programs aim to provide a comprehensive understanding of the regulatory process, covering areas like clinical trial management, drug safety, and post-market surveillance.
Johns Hopkins University, for example, offers a Master of Science in Regulatory Science. Their program focuses on training professionals to navigate the regulatory complexities of drugs, biologics, and medical devices.
The curriculum often includes courses on FDA regulations, international regulatory harmonization, and compliance strategies. Students learn to interpret regulations, prepare submissions, and interact with regulatory agencies like the FDA.
The University of Southern California (USC) also boasts a well-regarded program in regulatory science. USC emphasizes both theoretical knowledge and practical skills through case studies and simulations.
Varying Approaches and Focus Areas
While many programs share core elements, their emphasis can differ significantly. Some programs prioritize pharmaceutical regulations, while others focus on medical devices or a broader range of regulated products.
Some programs also incorporate a strong focus on international regulatory requirements. This is particularly relevant for professionals working in global companies.
The varying focuses reflect the diverse career paths within regulatory affairs. These career paths range from specializing in clinical trials to focusing on post-market surveillance.
Challenges and Opportunities
Despite the growing number of programs, challenges remain in ensuring consistent quality and relevance. Employers often seek candidates with practical experience and a deep understanding of specific regulatory areas.
Some industry professionals have expressed concerns about the practical application of knowledge gained in some academic settings. Bridging the gap between theory and practice is crucial for program success.
Opportunities exist to enhance programs through collaborations with industry partners. These collaborations could include internships, guest lectures, and joint research projects.
Dr. Emily Carter, a regulatory affairs consultant with Carter Regulatory Solutions, notes, "Universities need to engage more with industry to ensure their curricula reflect the current needs and challenges faced by regulatory professionals." She added, "This collaborative approach will produce more job-ready graduates."
The Role of Accreditation and Standardization
The lack of a standardized accreditation process for regulatory affairs programs presents another challenge. This makes it difficult for prospective students and employers to evaluate the quality of different programs.
The Regulatory Affairs Professionals Society (RAPS) offers certifications for individuals. However, RAPS does not accredit academic programs.
Some argue that a formal accreditation process could help improve program quality and ensure greater consistency across institutions. This would allow employers to better gauge the skills and knowledge of graduates.
"Standardization is key to ensuring that all graduates possess a foundational understanding of regulatory principles," says David Lee, a regulatory manager at MedTech Innovations. "Without it, employers must spend considerable time and resources training new hires on basic concepts."
Looking Ahead: The Future of Regulatory Affairs Education
The future of regulatory affairs education hinges on its ability to adapt to the evolving needs of the industry. This includes incorporating new technologies, addressing emerging regulatory challenges, and fostering greater collaboration between academia and industry.
Emphasis on practical skills, such as preparing regulatory submissions and interacting with regulatory agencies, will become increasingly important. Furthermore, programs will need to address the growing complexity of global regulatory landscapes.
As the demand for regulatory affairs professionals continues to grow, universities must invest in developing robust, relevant, and standardized programs. These programs should equip graduates with the skills and knowledge necessary to navigate the complexities of the regulatory world and contribute to the advancement of public health.