Salt Dissolving In Water Is A Physical Change.

For generations, students have learned that dissolving salt in water is a classic example of a physical change. This understanding, deeply ingrained in science education, reflects the observation that the salt crystals seemingly disappear, only to reappear when the water evaporates, leaving the salt behind, unchanged in its fundamental chemical composition.
However, recent discussions among educators and chemists highlight the importance of continually revisiting and refining how this concept is taught, focusing on accurately portraying the molecular interactions at play.
Understanding Physical Changes
A physical change is typically defined as a change in the form or appearance of a substance, but not its chemical composition. This means the substance is still the same substance, even though it might look different.
Examples often cited include melting ice (solid water to liquid water), boiling water (liquid water to gaseous water), and tearing paper. In each of these cases, the chemical identity of the water (H2O) or the paper (cellulose) remains unchanged.
The Case of Salt and Water: Key Details
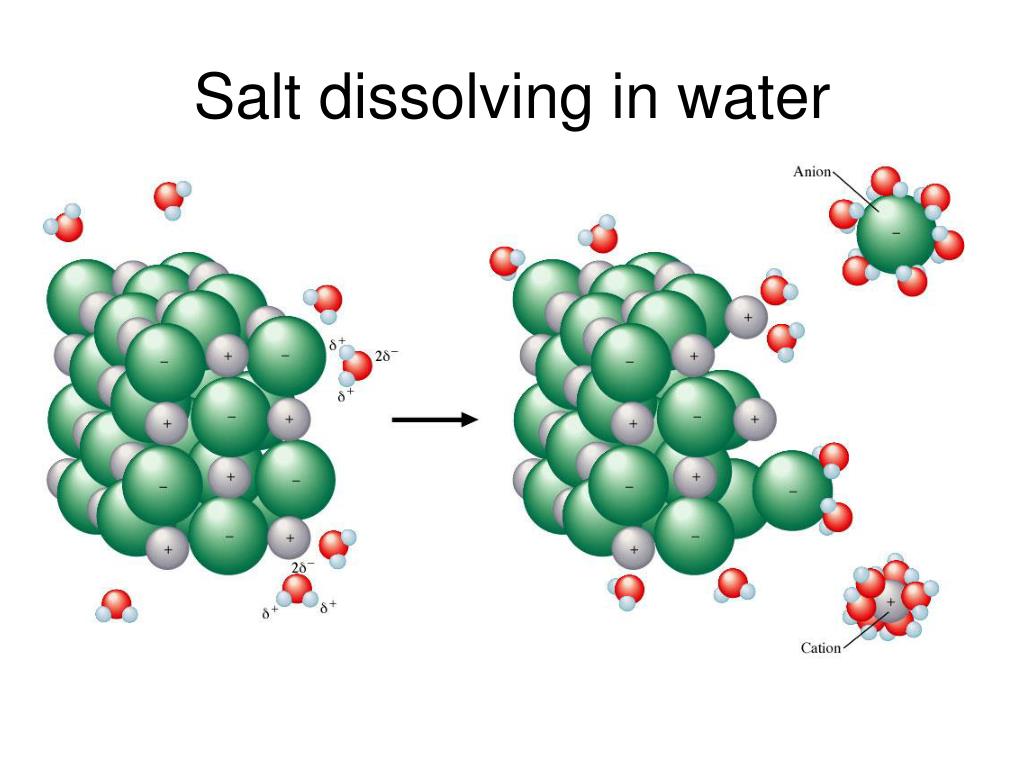
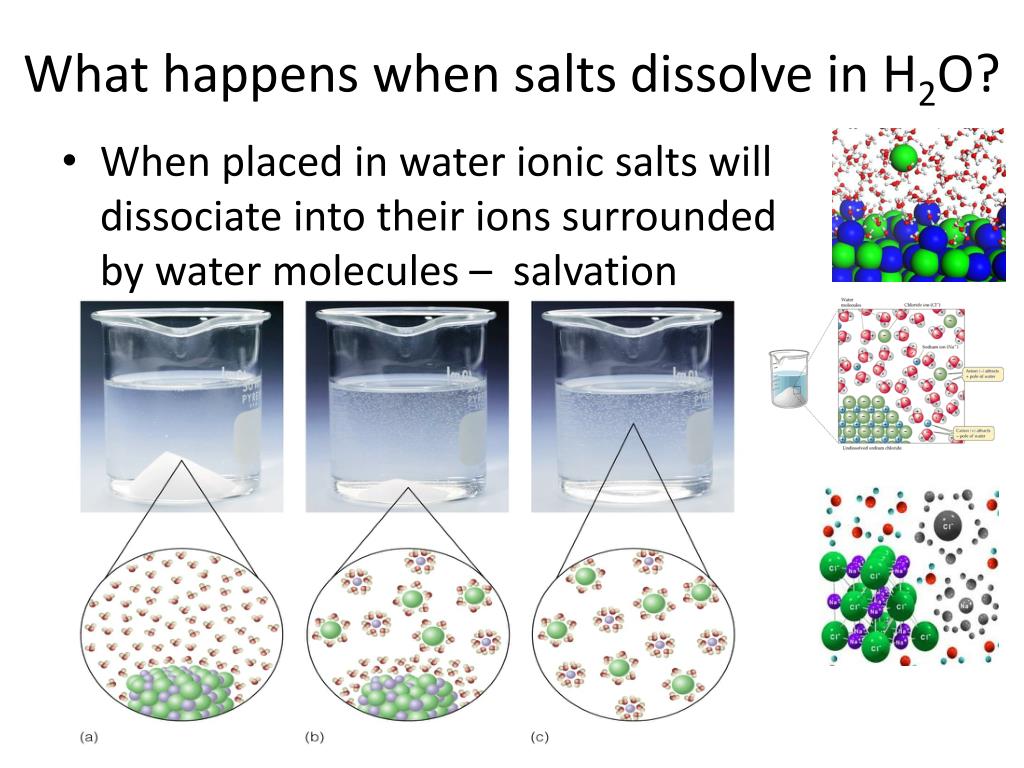
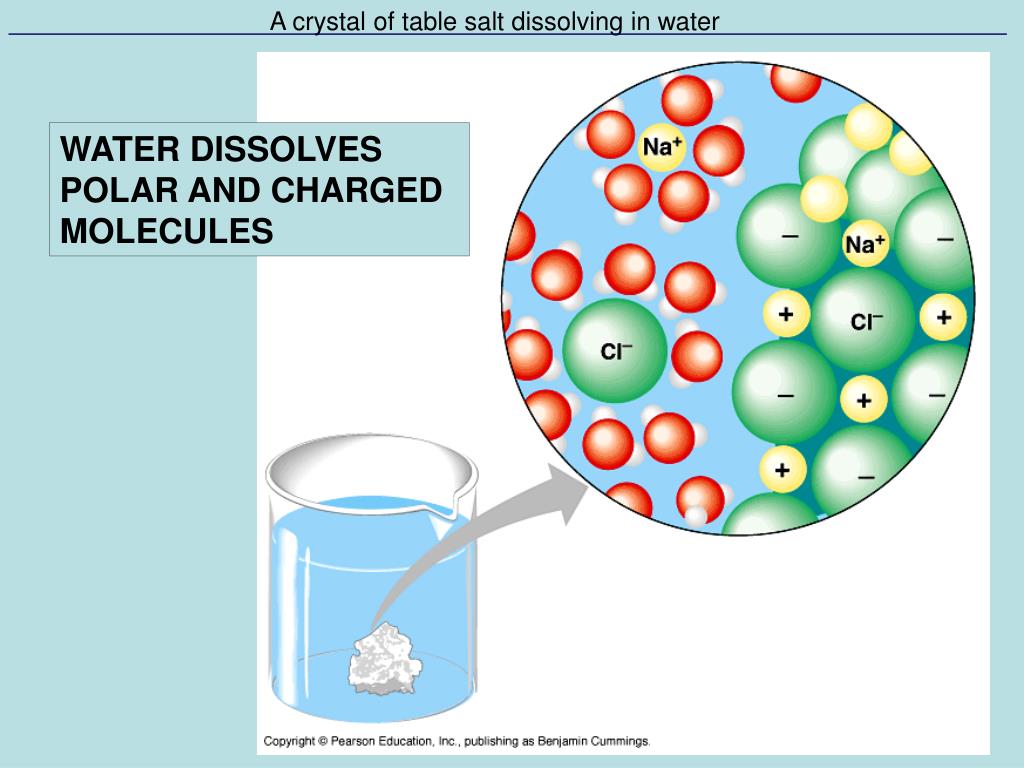
The widely accepted view of dissolving salt (sodium chloride, NaCl) in water involves the following steps. First, salt crystals are comprised of sodium (Na+) and chloride (Cl-) ions held together by strong electrostatic forces.
When salt is added to water, the water molecules, which are polar, interact with these ions. The negatively charged oxygen atoms in water are attracted to the positively charged sodium ions, and the positively charged hydrogen atoms are attracted to the negatively charged chloride ions.
These interactions weaken and eventually overcome the electrostatic forces holding the salt crystal together, causing the ions to dissociate and disperse throughout the water.
The key point, in terms of a physical change, is that the sodium and chloride ions are still sodium and chloride ions, and the water is still water. No new chemical species are formed.
Significance and Potential Impact
The significance of correctly understanding this process lies in building a solid foundation for more advanced chemical concepts. Misunderstandings at this basic level can lead to difficulties when students begin to study chemical reactions and equilibrium.
Furthermore, a precise understanding of dissolution is vital in various fields, from food science (understanding how salt impacts taste and preservation) to environmental science (analyzing salinity in water systems) and medicine (developing saline solutions for intravenous drips).
The ongoing discussions surrounding this topic highlight the dynamic nature of scientific understanding and the importance of critical thinking in science education.
Nuances and Ongoing Discussions
While dissolving salt in water is generally considered a physical change, it's important to acknowledge certain nuances that are often simplified in introductory science classes. For example, hydration shells form around the ions, changing their immediate environment.
"It's not just a simple dispersal; the ions interact strongly with the water molecules, forming hydration complexes,"
explained Dr. Anya Sharma, a chemistry professor at the University of California, Berkeley. These interactions, while not forming new covalent bonds, do influence the properties of the solution.
Some argue that these interactions could be considered a very mild form of chemical change, blurring the lines between physical and chemical processes. This is a debate that is usually reserved for more advanced chemistry courses.
Human-Interest Angle: A Teacher's Perspective
Mrs. Emily Carter, a high school science teacher with 15 years of experience, shares her perspective. "I've always taught it as a physical change, but I also try to emphasize that there's more to the story than meets the eye."
"I show them videos of the ions interacting with water molecules, so they understand it's not just vanishing. It's about visualising the invisible." This hands-on approach sparks curiosity in students, encouraging them to question and explore beyond the textbook definition.
Conclusion
The classification of dissolving salt in water as a physical change remains a fundamental concept in science education. While the process involves intricate molecular interactions, the essential chemical identity of the salt and water is retained.
Continued discourse among educators and scientists underscores the importance of presenting this concept with clarity and precision, equipping students with a solid understanding of the world around them.
The key takeaway is the need to continually refine how scientific principles are taught, ensuring they are both accessible and accurate, fostering a spirit of inquiry and critical thinking.