Stem Cell Therapy For Neuropathy Clinical Trials

Hope flares for neuropathy sufferers as groundbreaking stem cell therapy trials show promise. Early data suggests potential for pain reduction and nerve regeneration, offering a beacon for millions facing chronic suffering.
These clinical trials represent a critical step toward a potential cure for a condition that often leaves patients with limited treatment options. The focus is on rigorously testing the efficacy and safety of stem cell therapies to alleviate the debilitating symptoms of neuropathy.
Ongoing Clinical Trials: A Glimpse of Progress
Several institutions across the United States are currently involved in these pioneering studies. Mayo Clinic, University of Miami Miller School of Medicine, and Stanford University are among the leading centers actively recruiting participants. They all explore different approaches to stem cell delivery and treatment protocols.
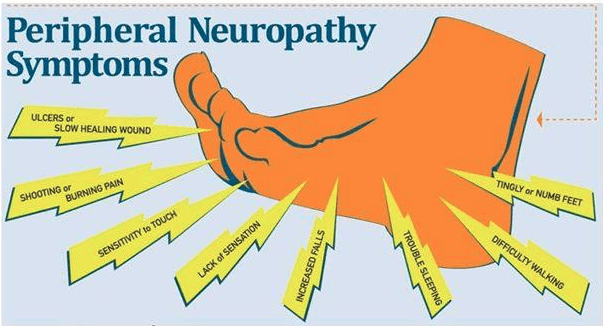
The what: clinical trials investigate the use of stem cells to repair damaged nerves in patients with various forms of neuropathy. This includes diabetic neuropathy, chemotherapy-induced peripheral neuropathy, and idiopathic neuropathy.
The who: the studies target adults diagnosed with confirmed neuropathy who meet specific inclusion criteria. Eligibility is based on factors such as disease severity, overall health, and prior treatment history.
The where: trials are underway at multiple sites across the US, with expansion to other countries potentially on the horizon. Each center has dedicated research teams overseeing the trials and providing patient care.
The when: enrollment is ongoing, with timelines varying depending on the specific trial protocol. Initial results from some studies are expected within the next 12-24 months.
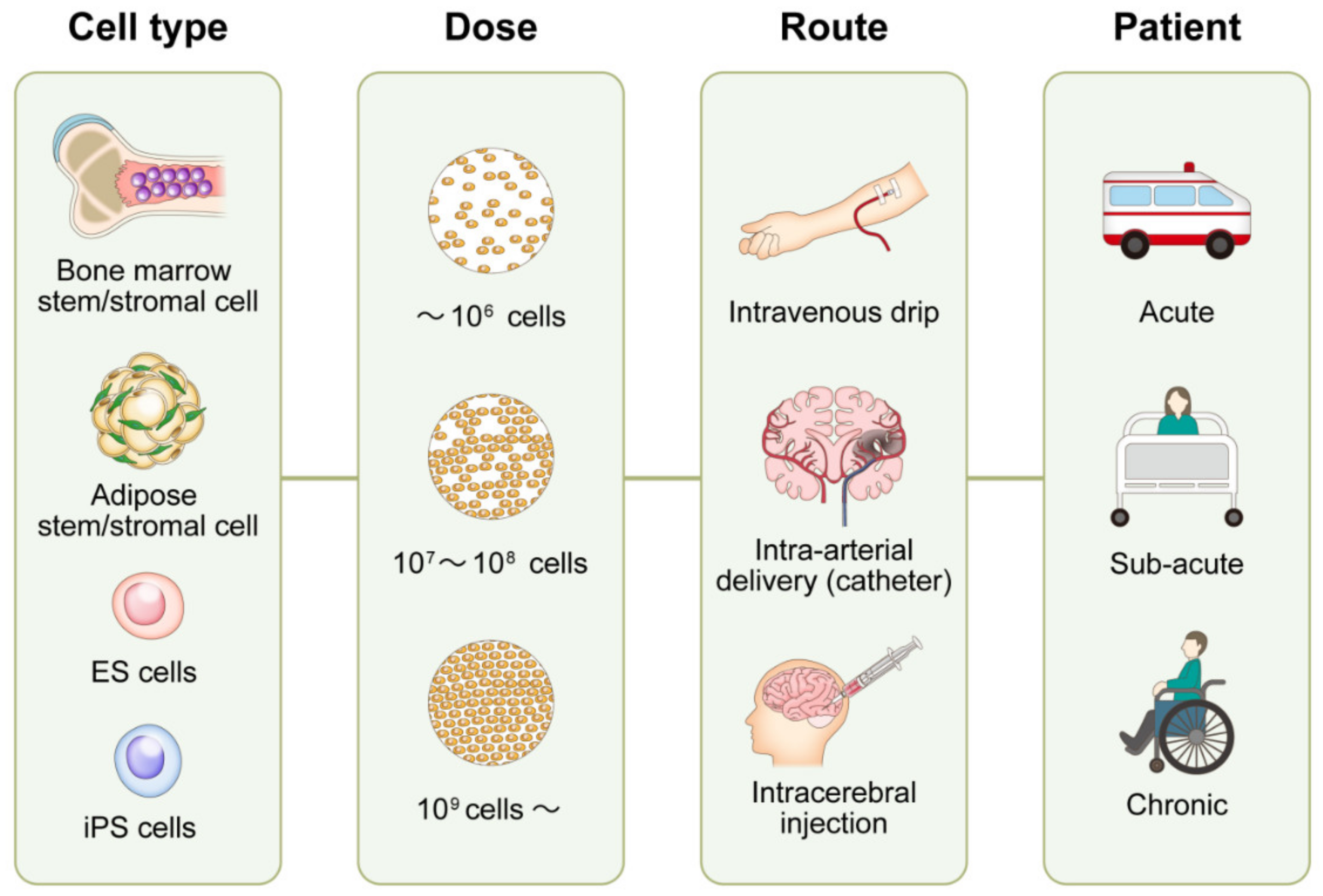
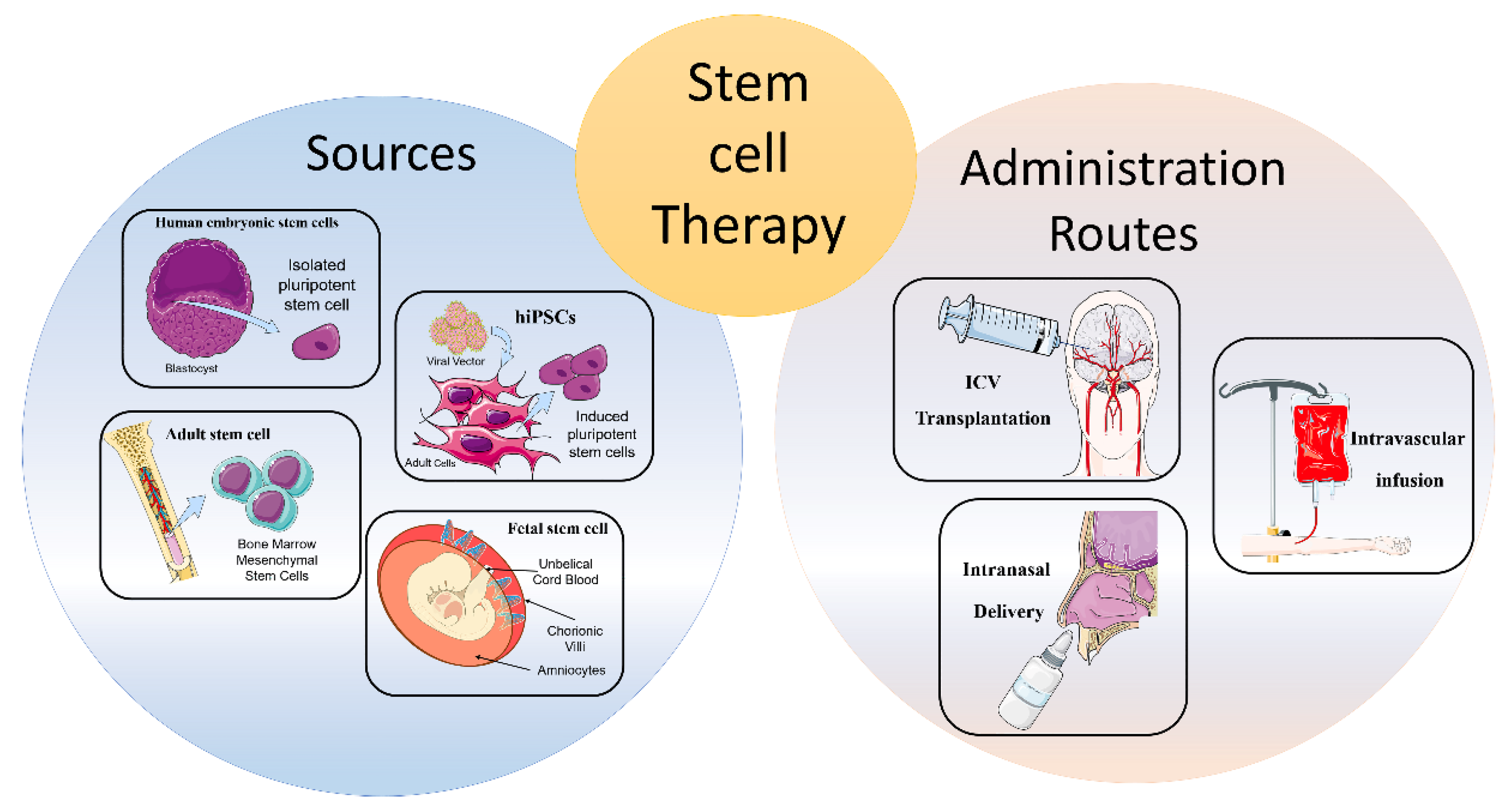
Types of Stem Cells and Delivery Methods
Researchers are exploring various types of stem cells. These include autologous stem cells (derived from the patient's own body) and allogeneic stem cells (derived from a donor).
Common delivery methods involve intravenous infusion or direct injection into the affected area. The chosen method depends on the type of stem cell and the location of the nerve damage.
Intravenous infusion allows for systemic distribution of stem cells throughout the body. Direct injection delivers stem cells directly to the damaged nerves, theoretically maximizing their therapeutic effect.
Preliminary Findings: Signs of Hope
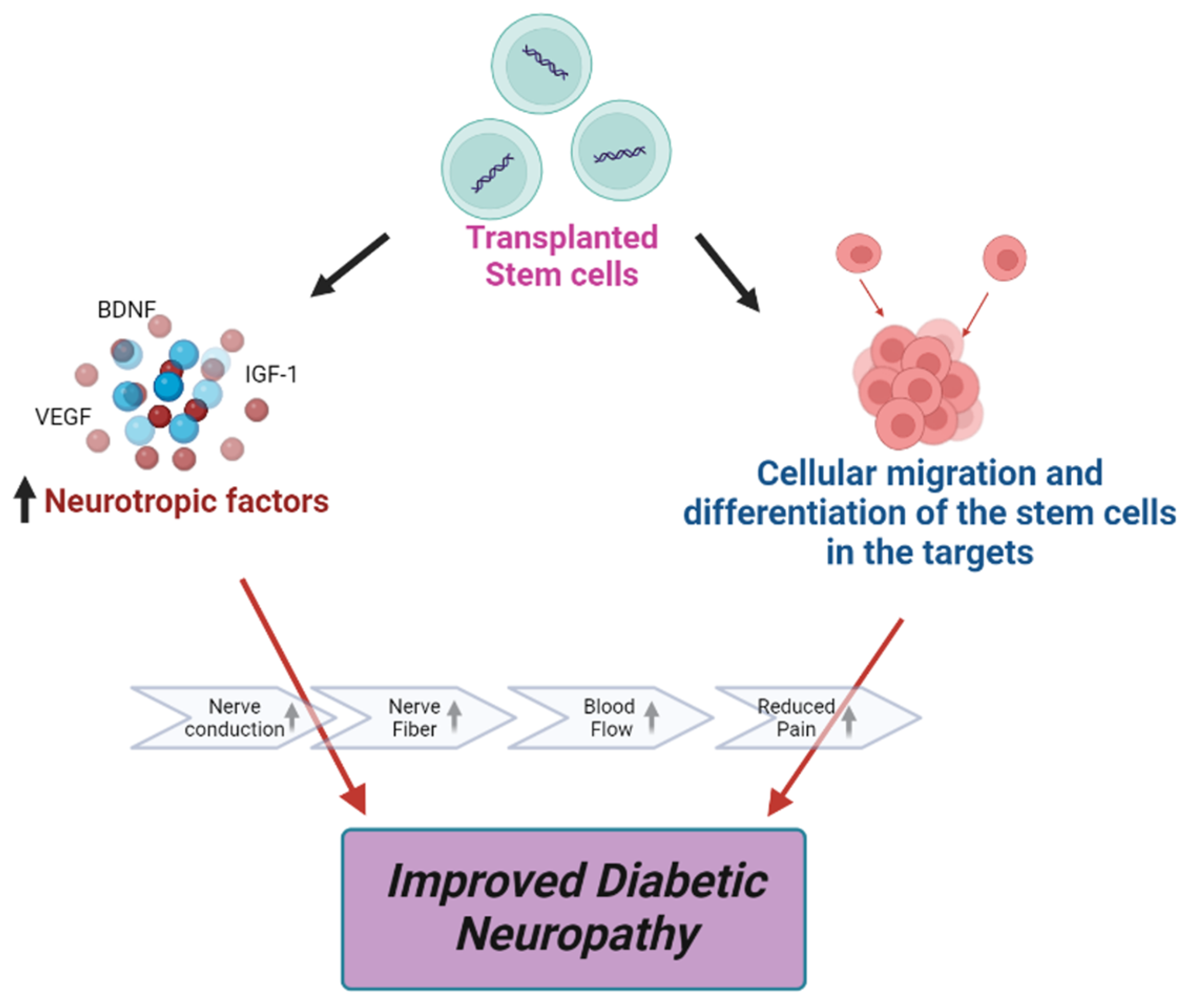
Early data from some trials are encouraging, although definitive conclusions are still pending. Some patients have reported a noticeable reduction in pain, improved sensation, and increased mobility.
Objective measures, such as nerve conduction studies, are also being used to assess nerve regeneration. Early findings suggest that stem cell therapy may promote nerve repair in some cases.
Dr. Eleanor Vance, lead investigator at the University of Miami, stated, "We are observing promising signs of nerve regeneration in a subset of patients, which is incredibly encouraging. However, more rigorous studies are needed to confirm these findings."
Safety and Efficacy: The Core Focus
Patient safety remains the top priority in all ongoing trials. Researchers are meticulously monitoring participants for any adverse effects associated with stem cell therapy.
Efficacy is being evaluated using standardized pain scales, neurological examinations, and nerve conduction studies. The goal is to determine whether stem cell therapy provides a clinically significant improvement compared to standard treatments.
Robust data analysis is crucial to validate the therapeutic benefits of stem cell therapy. Rigorous statistical methods are employed to minimize bias and ensure the reliability of the results.
Challenges and Future Directions
Significant challenges remain, including optimizing stem cell delivery and ensuring long-term efficacy. The long-term effects of stem cell therapy on nerve regeneration need further investigation.
Researchers are also exploring combination therapies that integrate stem cells with other treatments, such as physical therapy and medication. This approach aims to maximize the therapeutic potential of stem cell therapy.
Further research is needed to identify the optimal type of stem cell for treating different types of neuropathy. Understanding the mechanisms by which stem cells promote nerve regeneration is also critical.
The Road Ahead
While these clinical trials offer a glimmer of hope, it is crucial to maintain realistic expectations. Stem cell therapy is still in its early stages of development, and much work remains to be done.
Patients interested in participating in clinical trials should consult with their healthcare providers. Thorough evaluation is necessary to determine eligibility and ensure that participation is appropriate.
Continued investment in stem cell research is essential to accelerate the development of effective treatments for neuropathy. The next phase of trials will focus on larger patient populations and longer follow-up periods to assess the long-term benefits and risks of stem cell therapy.