Stopping Invega Sustenna Cold Turkey

The long-acting injectable antipsychotic medication, Invega Sustenna, used in the treatment of schizophrenia and schizoaffective disorder, requires careful management, especially when considering discontinuation. Abrupt cessation, often referred to as "stopping cold turkey," has raised concerns among medical professionals and patients alike due to the potential for adverse effects and symptom relapse.
This article examines the implications of discontinuing Invega Sustenna without a gradual tapering schedule, drawing from medical literature and expert opinions to provide a comprehensive overview of the risks and considerations.
Understanding Invega Sustenna and Its Mechanism
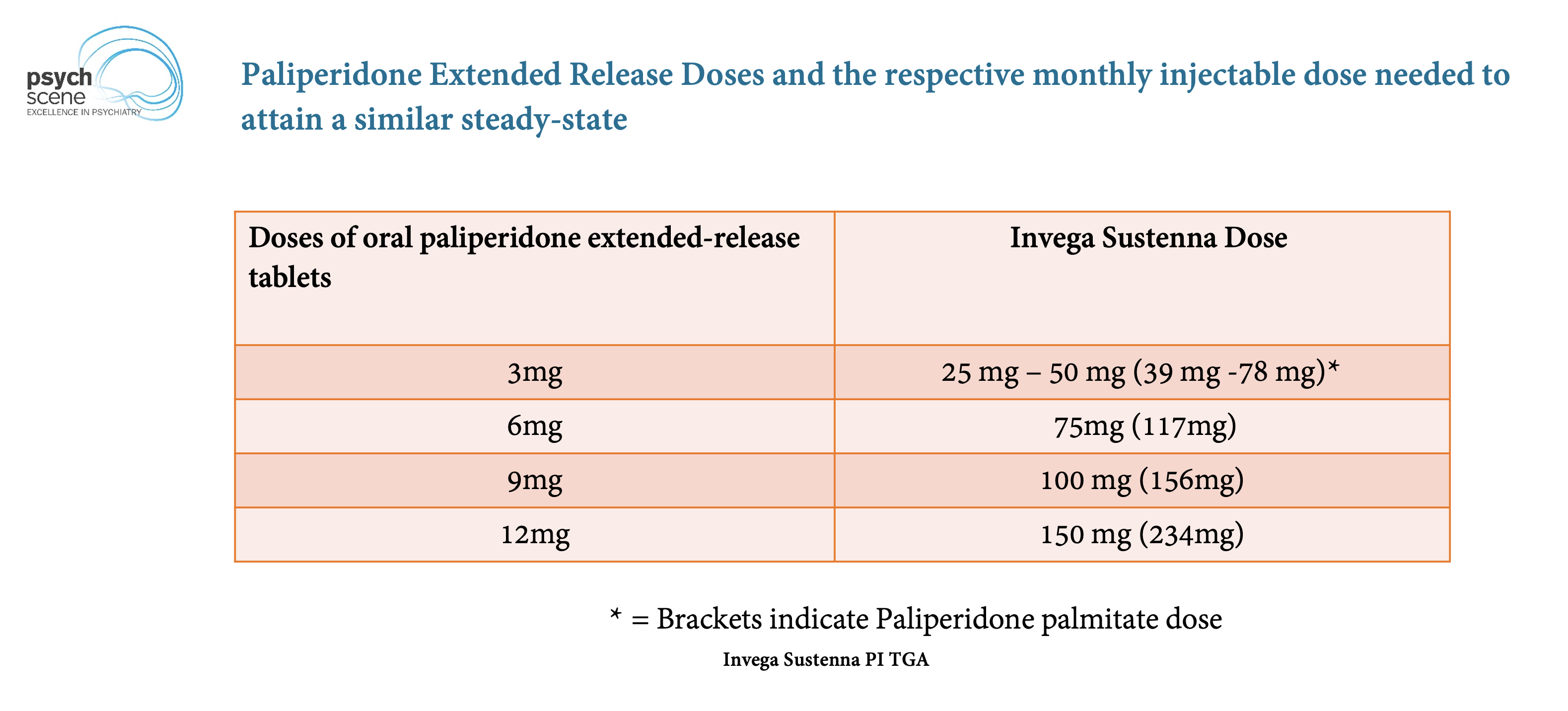
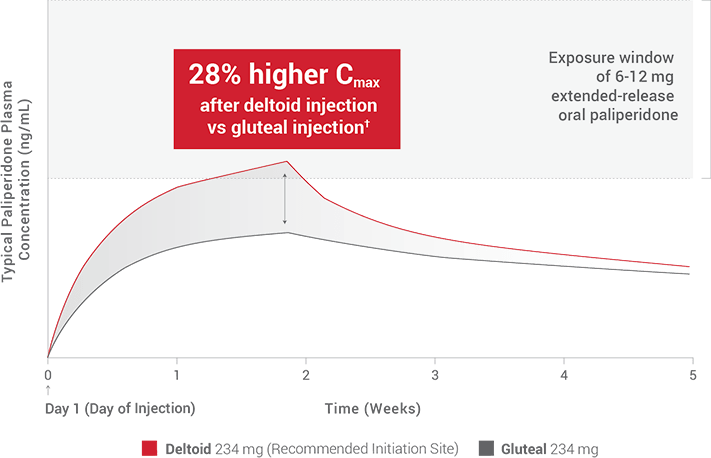
Invega Sustenna is a brand name for paliperidone palmitate, an atypical antipsychotic administered via intramuscular injection. The medication works by affecting neurotransmitters in the brain, primarily dopamine and serotonin, helping to regulate mood, thought, and behavior.
Its extended-release formulation allows for less frequent dosing compared to oral medications, improving adherence for some individuals.
Why Tapering is Generally Recommended
Medical guidelines generally advise against abruptly stopping antipsychotic medications, including Invega Sustenna. The brain adapts to the presence of the medication over time, and sudden withdrawal can disrupt this equilibrium.
This disruption can lead to a range of withdrawal symptoms and a higher risk of relapse.
Potential Risks of Cold Turkey Discontinuation
Stopping Invega Sustenna cold turkey can trigger several adverse effects. One significant concern is the potential for antipsychotic withdrawal symptoms, which can include nausea, vomiting, dizziness, anxiety, insomnia, and sweating.
Furthermore, there is a heightened risk of relapse of the underlying psychotic disorder. Studies have shown that abrupt discontinuation significantly increases the likelihood of symptoms returning, sometimes more intensely than before, a phenomenon known as rebound psychosis.
According to a 2016 study published in the Journal of Clinical Psychiatry, patients who abruptly discontinued antipsychotics were more likely to experience relapse and hospitalization compared to those who tapered off the medication gradually.
"Abrupt discontinuation of antipsychotics can destabilize the brain's neurochemical balance, increasing the risk of relapse and withdrawal symptoms," explains Dr. Anya Sharma, a psychiatrist specializing in schizophrenia treatment.
In some cases, discontinuation may lead to a severe condition known as neuroleptic malignant syndrome (NMS), although this is rare. NMS is characterized by fever, muscle rigidity, altered mental status, and autonomic dysfunction and requires immediate medical attention.
Factors Influencing Discontinuation Risks
The risk associated with stopping Invega Sustenna cold turkey can vary depending on several factors. These include the individual's dosage, duration of treatment, and overall health status.
Individuals with a longer history of psychotic illness or those who have experienced previous relapses may be at a higher risk.
The Importance of Medical Supervision
Discontinuation of Invega Sustenna should always be done under the guidance of a qualified healthcare professional. A doctor can assess the individual's specific situation and develop a tapering schedule that minimizes the risk of adverse effects.
The tapering process typically involves gradually reducing the dosage over several weeks or months, allowing the brain to adapt to the change.
During this period, close monitoring is essential to detect any signs of withdrawal or relapse. "Careful monitoring is crucial when discontinuing any antipsychotic medication," emphasizes Dr. Ben Carter, a clinical pharmacist. "Regular check-ups with a healthcare provider allow for timely intervention if any issues arise."
Alternative treatment options, such as switching to a different medication or exploring non-pharmacological therapies, can also be considered during the tapering process.
Patient Experiences and Support
Many individuals who have attempted to discontinue Invega Sustenna without medical supervision have reported challenging experiences. Online forums and support groups are filled with anecdotal accounts of withdrawal symptoms and relapse, highlighting the importance of seeking professional help.
It’s crucial for patients to have open communication with their healthcare providers and to actively participate in the decision-making process regarding their treatment.
Support groups and mental health organizations can provide valuable resources and emotional support during this transition.
Conclusion
While discontinuing Invega Sustenna may be a consideration for some individuals, stopping cold turkey poses significant risks. The potential for withdrawal symptoms, relapse, and other adverse effects underscores the importance of medical supervision and a carefully managed tapering process.
Patients should consult with their healthcare providers to develop a safe and effective discontinuation plan tailored to their individual needs.
Ultimately, a collaborative approach between the patient and their medical team is essential for achieving the best possible outcome.