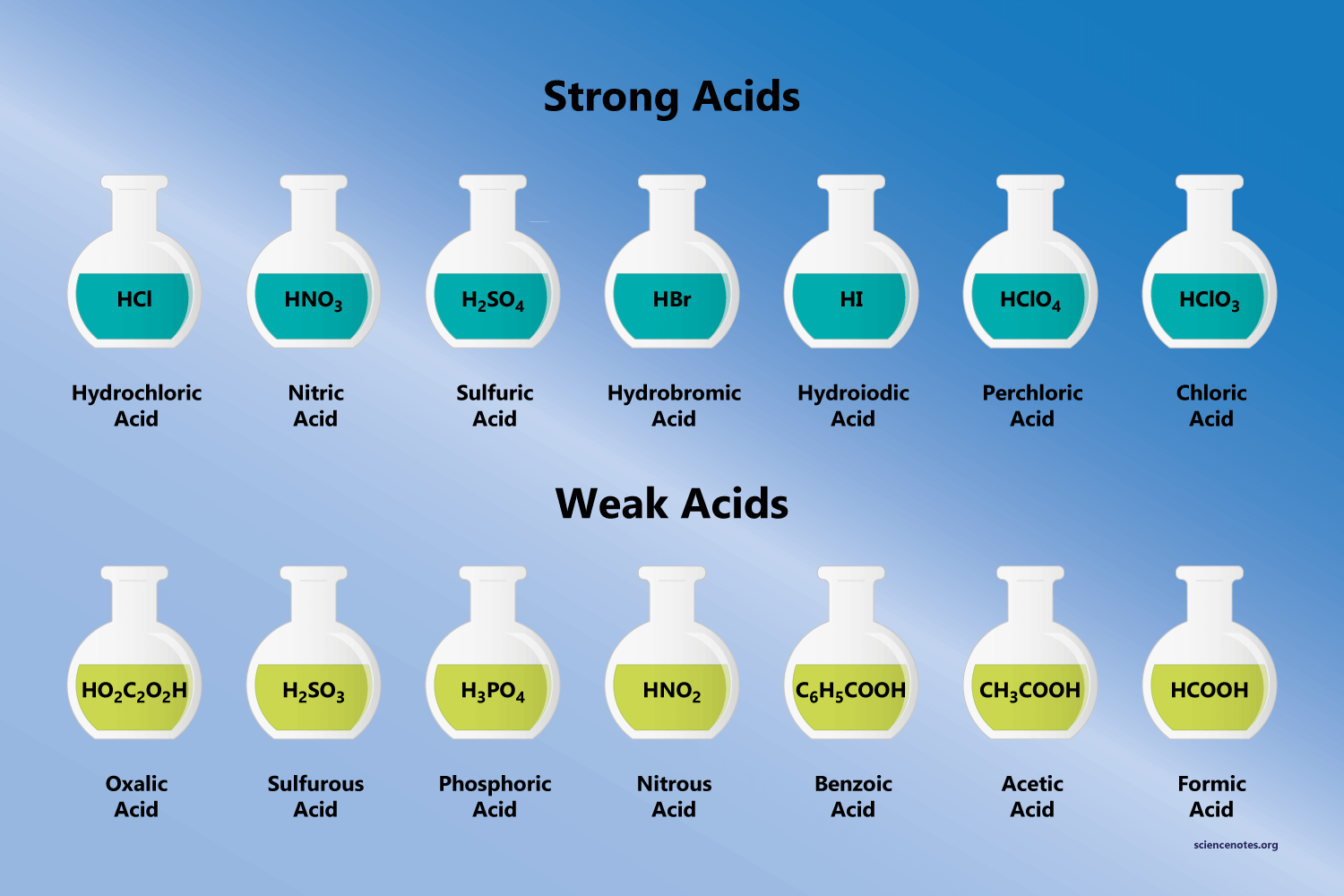
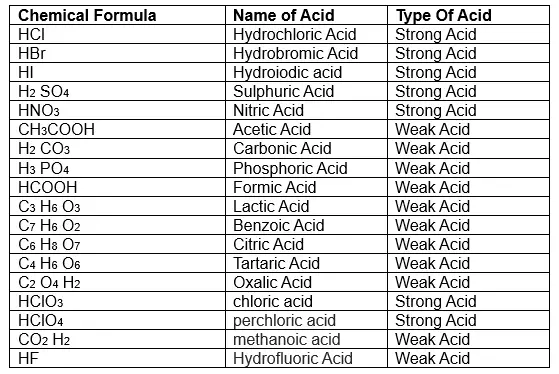
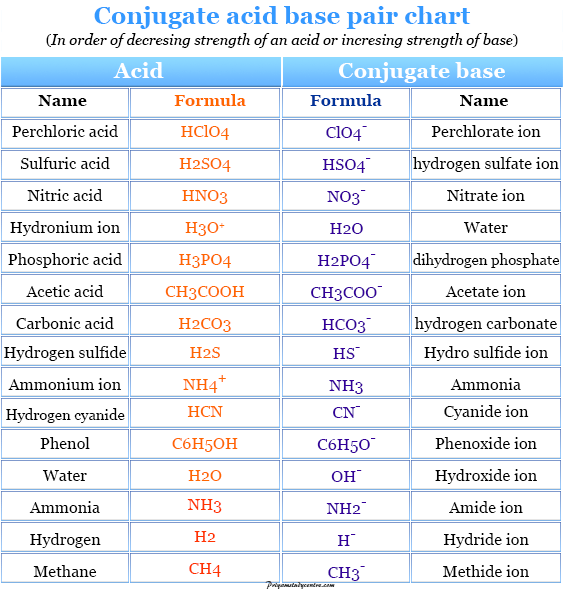
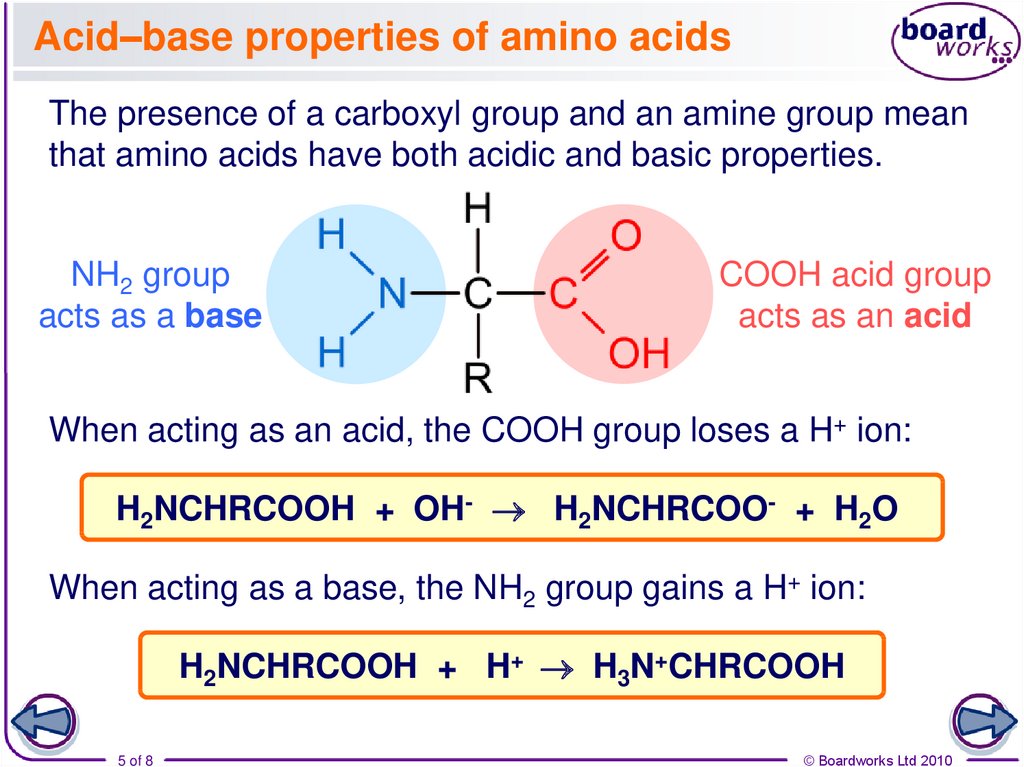
The Chemical Group That Acts As An Acid Is The
:max_bytes(150000):strip_icc()/common-acids-and-chemical-structures-603645_FINAL-54e6b0b3351b49dbb6cef54fd4817404.png)
The pervasive presence of acidity touches nearly every facet of modern life, from the industrial processes that manufacture crucial materials to the delicate biological reactions that sustain life itself. Yet, beneath the surface of common knowledge lies a critical question: What is the specific chemical group responsible for acidic behavior? The answer has profound implications for understanding chemical reactions, developing new technologies, and addressing environmental challenges.
This article will explore the chemical group responsible for acidic behavior, focusing on its structure, function, and impact across various fields. We will delve into the scientific understanding of acids and their properties, clarifying the role of this key chemical entity. The analysis will draw upon expert opinions, scientific research, and real-world examples to provide a comprehensive overview of this essential concept in chemistry.
The Acidic Culprit: The Hydrogen Ion (H+)
The chemical group primarily responsible for acidic behavior is the hydrogen ion (H+). This seemingly simple entity holds the key to understanding acidity in chemical systems. Acids, by definition, are substances that donate or release hydrogen ions in solution.
The Brønsted-Lowry definition of an acid specifically identifies it as a proton (H+) donor. When an acid dissolves in water, it releases H+ ions, increasing the concentration of H+ in the solution.
How Hydrogen Ions Create Acidity
The increased concentration of H+ ions is what characterizes an acidic solution. These ions readily react with other molecules, leading to the properties we associate with acids.
For example, acids can corrode metals, sour the taste of foods, and change the color of certain indicators. These reactions are all driven by the reactivity of the hydrogen ion.
The Role of Functional Groups
While the hydrogen ion itself is responsible for acidity, certain functional groups facilitate its release. Functional groups containing hydrogen atoms bonded to electronegative atoms, like oxygen or halogens, are particularly prone to acting as proton donors.
The electronegativity of these atoms pulls electron density away from the hydrogen atom, making it easier to be released as an H+ ion. This effect is crucial for understanding the acidity of various organic and inorganic compounds.
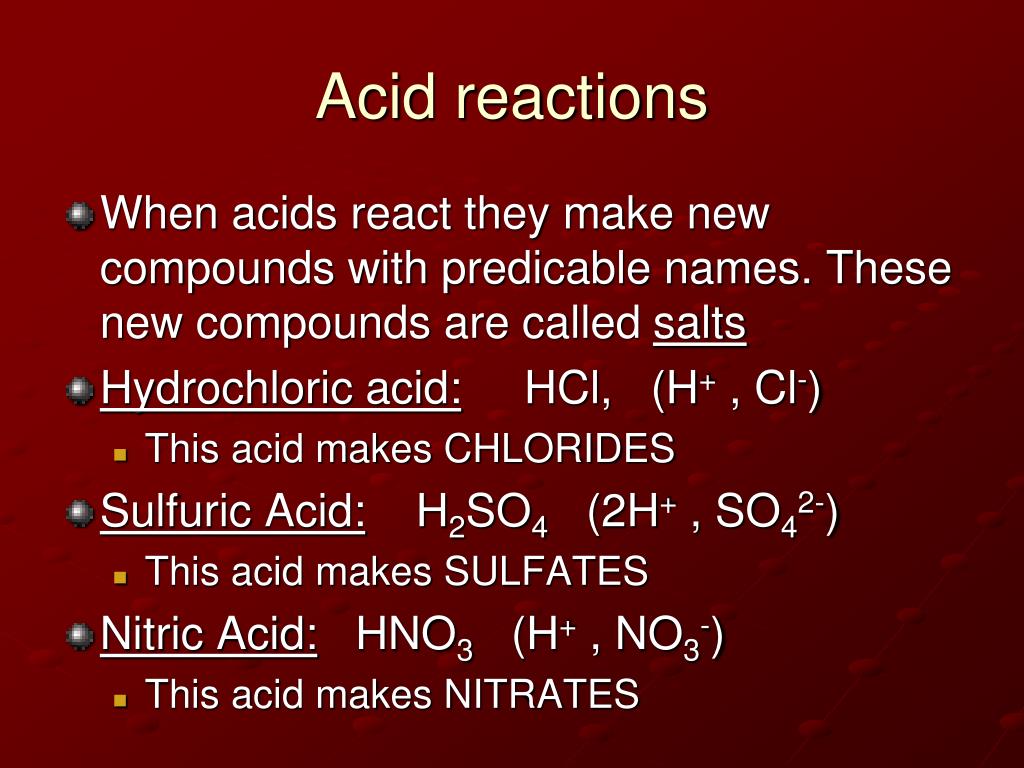
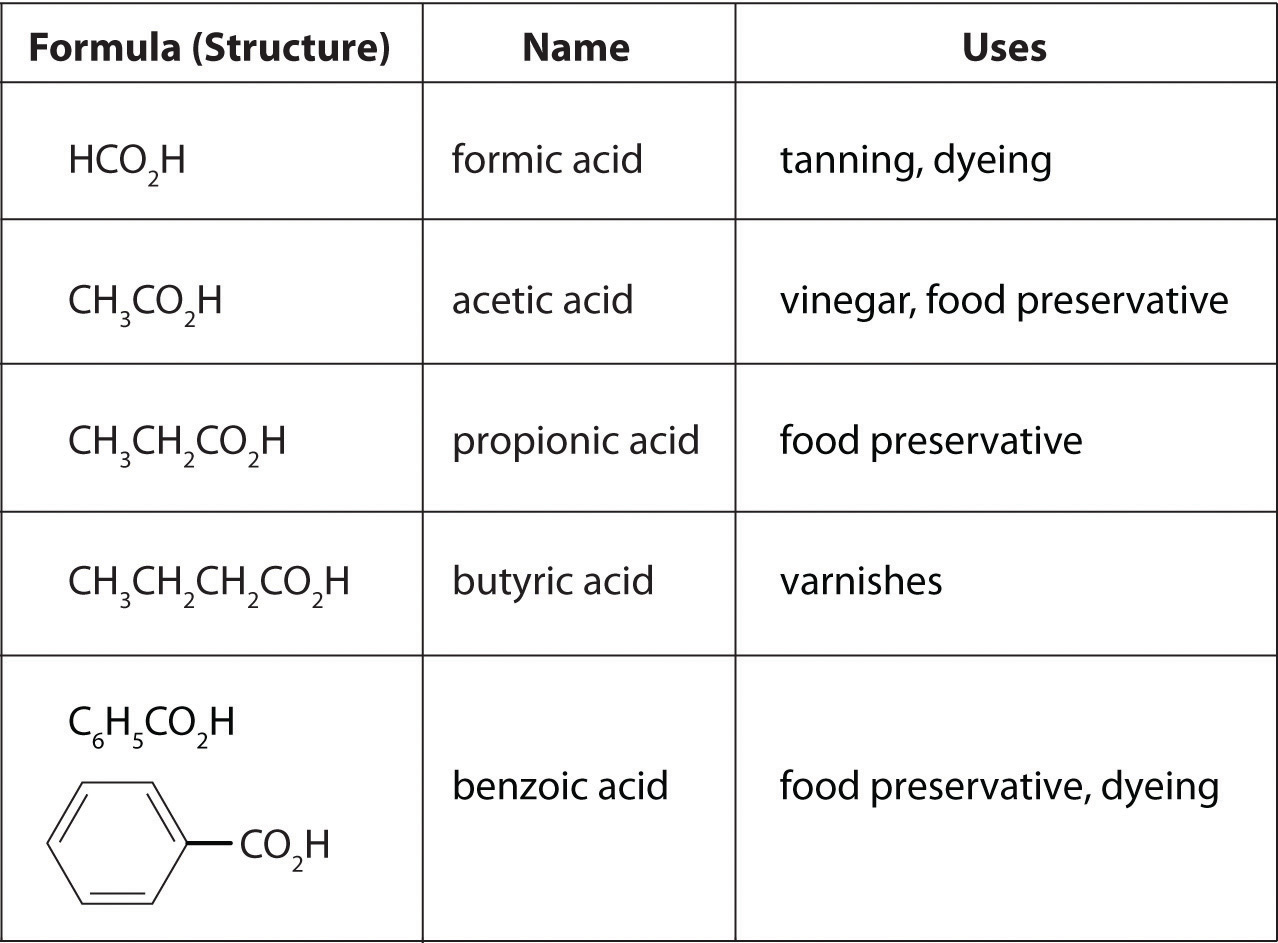
Carboxylic Acids: A Prime Example
Carboxylic acids are a common example of organic compounds that exhibit acidity due to the presence of the carboxyl group (-COOH). The hydrogen atom attached to the oxygen atom in the carboxyl group is relatively acidic.
This is because the oxygen atom is electronegative, and the resulting carboxylate anion is stabilized by resonance. This resonance stabilization makes the release of H+ more favorable.
Measuring Acidity: pH and pKa
The acidity of a solution is quantitatively measured using the pH scale. The pH scale ranges from 0 to 14, with values below 7 indicating acidic conditions.
A lower pH value corresponds to a higher concentration of H+ ions and, therefore, greater acidity. The pH scale is logarithmic, meaning each unit change represents a tenfold change in H+ concentration.
pKa: A Measure of Acid Strength
The pKa value is another important measure of acidity, specifically reflecting the strength of an acid. It is the negative logarithm of the acid dissociation constant (Ka), which quantifies the extent to which an acid dissociates in solution.
A lower pKa value indicates a stronger acid, meaning it readily releases H+ ions. The pKa value is a characteristic property of a particular acid.
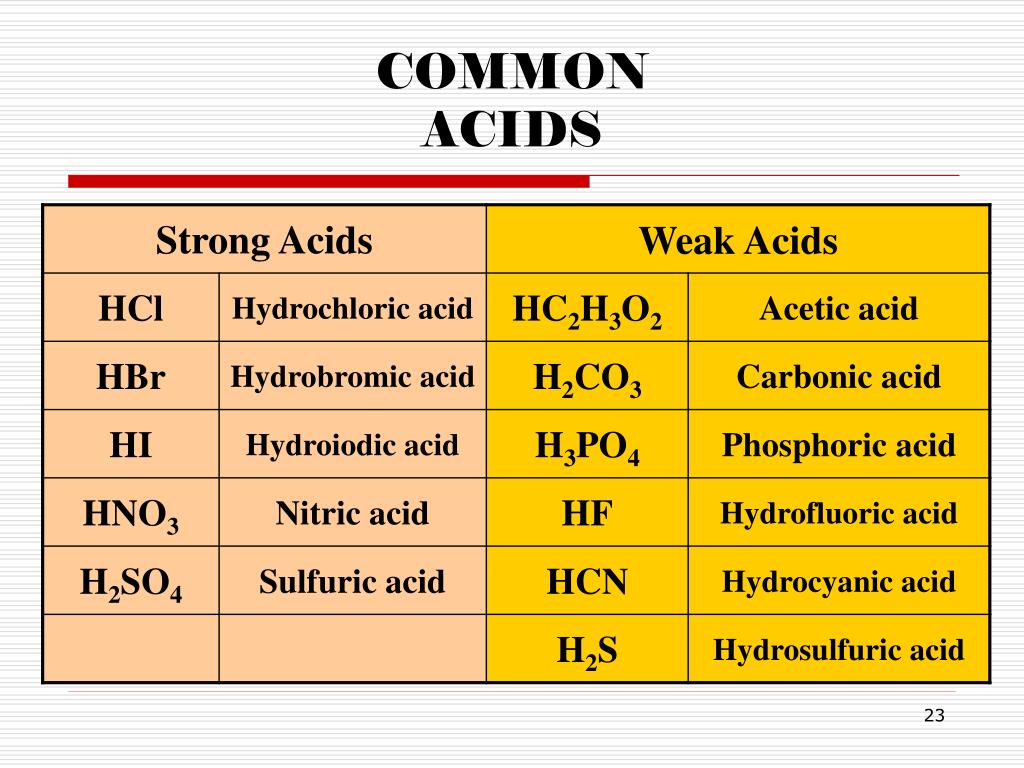
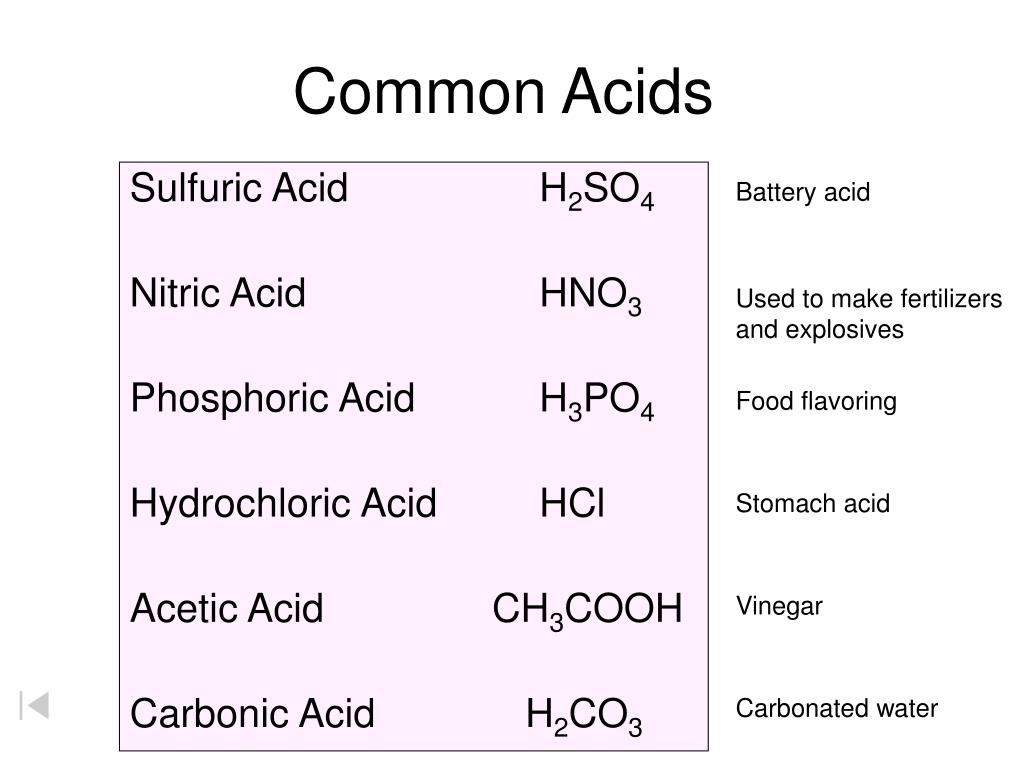
Acidity in Different Contexts
The concept of acidity plays a vital role in numerous fields. From industrial chemistry to biological systems, understanding the behavior of acids is essential.
Here are some examples of where acidity is crucial:
- Industrial Chemistry: Acids are used as catalysts, reactants, and solvents in various industrial processes, including the production of plastics, fertilizers, and pharmaceuticals.
- Biological Systems: The pH balance is critical for maintaining the proper functioning of biological systems. Enzymes, for example, have optimal activity within a specific pH range.
- Environmental Science: Acid rain, caused by atmospheric pollutants like sulfur dioxide and nitrogen oxides, can damage ecosystems and infrastructure.
Addressing the Challenges of Acidity
While acids are essential, their improper use or release can pose significant challenges. Acid rain is a prime example of the environmental consequences of uncontrolled acidity.
Furthermore, strong acids can be corrosive and dangerous, requiring careful handling and disposal. Ongoing research aims to develop safer and more sustainable alternatives to traditional acids.
Sustainable Solutions and Future Directions
Researchers are exploring alternative methods for achieving acidity in chemical reactions, such as using solid acid catalysts. These catalysts offer several advantages, including recyclability and reduced waste generation.
Furthermore, efforts are being made to develop new materials that are resistant to acidic corrosion. These advancements will contribute to a more sustainable and safer future.
Conclusion
The hydrogen ion (H+) is the chemical entity responsible for acidic behavior. Understanding the role of H+ ions and the functional groups that facilitate their release is fundamental to chemistry. From industrial processes to biological systems, acidity plays a crucial role, and ongoing research aims to address the challenges and promote sustainable solutions associated with its use and management. As we continue to explore the intricate details of chemistry, a firm grasp of the concepts surrounding acidity remains essential for innovation and progress.