The Dense Center Of An Atom

For over a century, the atomic nucleus has remained a focal point of intense scientific scrutiny. Within this incredibly small space lies the key to understanding the fundamental forces shaping our universe. Recent advancements in experimental techniques and theoretical modeling are now providing unprecedented insights into the nucleus's complex structure and behavior, revealing mysteries that could reshape our understanding of matter itself.
This article delves into the ongoing research efforts aimed at unraveling the secrets of the atomic nucleus. It will explore the forces at play within this dense core, the particles that constitute it, and the implications of this knowledge for fields ranging from energy production to medical imaging. This will encompass perspectives from leading physicists, groundbreaking experiments, and the ongoing quest to build a comprehensive model of the nucleus.
The Nucleus: A Realm of Immense Density
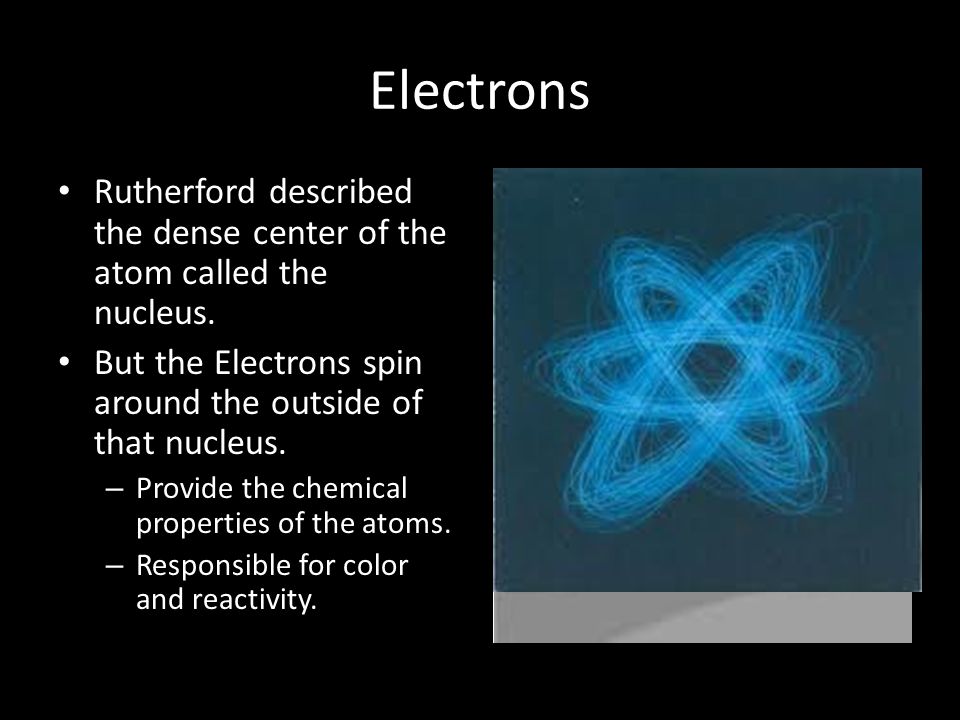
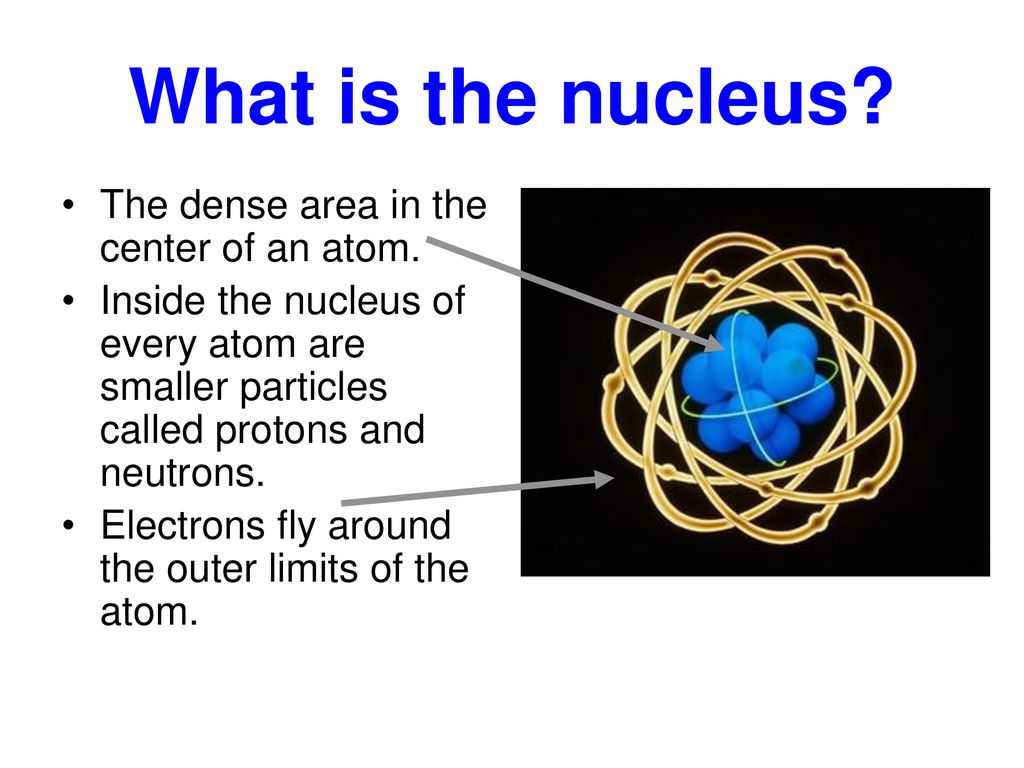
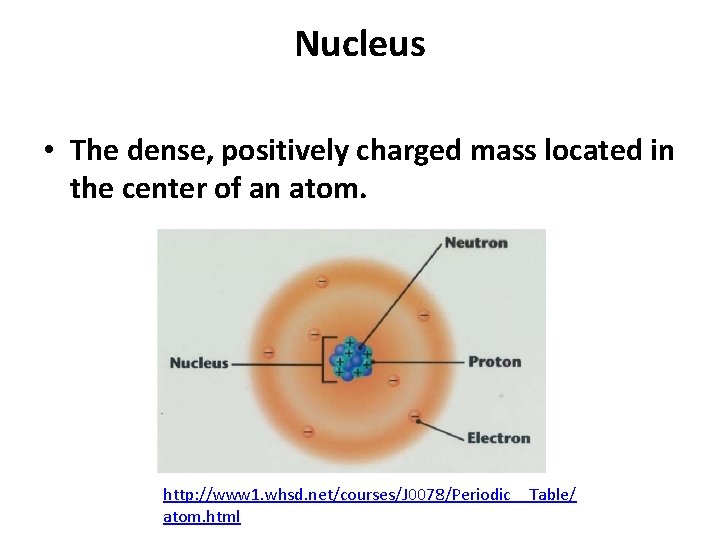
At the heart of every atom, a space approximately 100,000 times smaller than the atom itself, lies the nucleus. It contains virtually all of the atom’s mass.
This incredible density is a direct consequence of the strong nuclear force, one of the four fundamental forces in nature. This force overcomes the electrostatic repulsion between positively charged protons, binding them together alongside neutral neutrons.
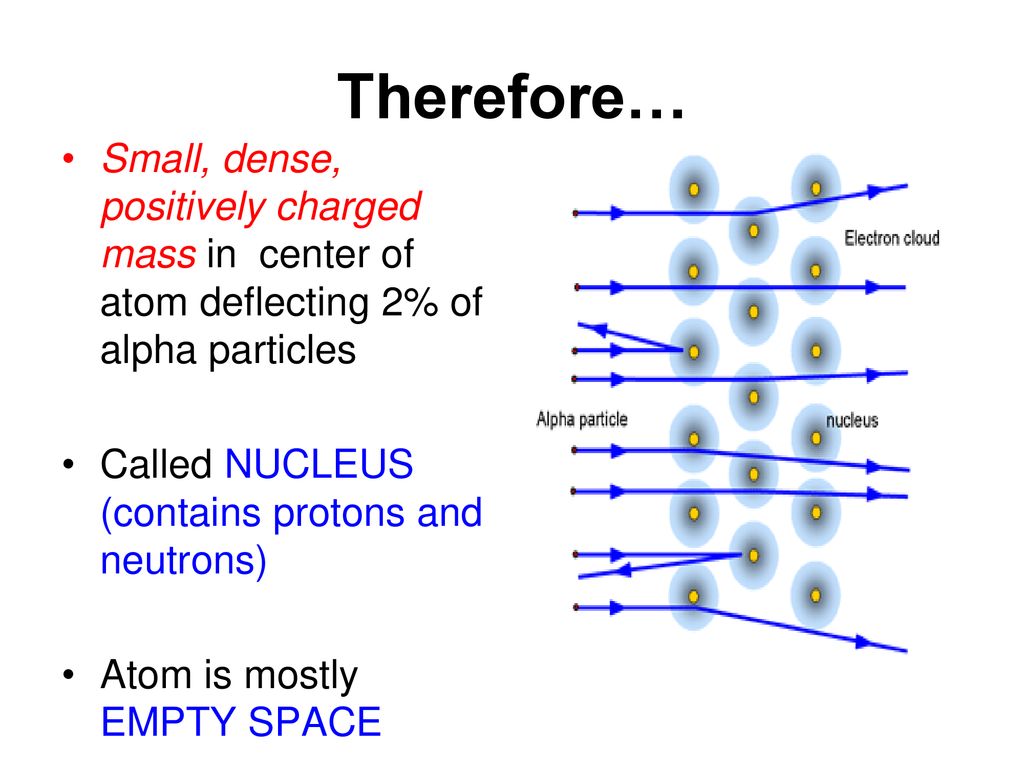
The discovery of the nucleus is attributed to Ernest Rutherford's gold foil experiment in 1911. His experiment demonstrated that atoms have a small, dense, positively charged core.
Protons and Neutrons: The Building Blocks
The nucleus consists primarily of protons and neutrons, collectively known as nucleons. Protons, with their positive charge, determine the element's identity.
The number of neutrons influences the isotope of that element. Isotopes of an element have the same number of protons but different numbers of neutrons.
Understanding the arrangement and interactions of protons and neutrons within the nucleus is paramount to predicting the stability and behavior of atomic nuclei. Research institutions like Oak Ridge National Laboratory conduct extensive work in this area.
The Strong Nuclear Force: A Complex Interaction
The strong nuclear force, mediated by particles called gluons, is responsible for holding the nucleus together. It is significantly stronger than the electromagnetic force at short distances.
However, its strength diminishes rapidly with increasing distance. This explains why nuclei become unstable as they grow larger.
Scientists are constantly refining their understanding of this force through experiments using particle accelerators and advanced theoretical models.
"Our understanding of the strong force is constantly evolving as we probe the nucleus at ever-smaller scales," says Dr. Eleanor Vance, a nuclear physicist at Argonne National Laboratory.
Unveiling Nuclear Structure: Experimental Approaches
Experimental nuclear physics employs a variety of techniques to probe the structure of the nucleus. These techniques range from scattering experiments to spectroscopic measurements.
High-energy particle accelerators, such as the Large Hadron Collider (LHC) at CERN and the Relativistic Heavy Ion Collider (RHIC) at Brookhaven National Laboratory, are crucial tools in this endeavor.
By colliding beams of heavy ions, scientists can create extreme conditions similar to those that existed in the early universe, providing insights into the fundamental properties of nuclear matter.
Theoretical Models: Simulating the Nucleus
Theoretical nuclear physics plays a vital role in interpreting experimental results and developing predictive models of nuclear behavior. Several theoretical models exist, each with its strengths and limitations.
The shell model describes the arrangement of nucleons in energy levels, analogous to electron shells in atoms. The liquid drop model treats the nucleus as a drop of incompressible fluid.
More sophisticated models, such as ab initio calculations, attempt to solve the fundamental equations of quantum chromodynamics (QCD) to describe nuclear interactions from first principles. These are computationally intensive.
Radioactivity and Nuclear Decay: Unstable Nuclei
Some nuclei are inherently unstable and undergo radioactive decay, emitting particles or energy to transform into a more stable configuration. There are several types of radioactive decay.
Alpha decay involves the emission of an alpha particle (a helium nucleus). Beta decay involves the emission of an electron or positron.
Gamma decay involves the emission of a gamma ray (a high-energy photon). Understanding these decay processes is crucial for applications in nuclear medicine and nuclear energy. The International Atomic Energy Agency (IAEA) plays a key role in monitoring and regulating radioactive materials worldwide.
Applications of Nuclear Physics: From Energy to Medicine
The knowledge gained from studying the atomic nucleus has led to numerous technological advancements. Nuclear energy, though controversial, provides a significant portion of the world's electricity.
Nuclear medicine utilizes radioactive isotopes for diagnostic imaging and cancer treatment. Technologies such as PET (Positron Emission Tomography) scans and radiation therapy rely on understanding nuclear decay processes.
Furthermore, nuclear techniques are employed in various fields, including materials science, archaeology, and environmental monitoring.
The Future of Nuclear Research: Exploring the Unknown
The study of the atomic nucleus remains an active and vibrant field of research. Future experiments and theoretical developments promise to shed further light on the structure, behavior, and fundamental properties of nuclear matter.
New facilities, such as the Facility for Rare Isotope Beams (FRIB) at Michigan State University, will enable scientists to study exotic nuclei with extreme neutron-to-proton ratios, expanding our understanding of nuclear stability.
Ultimately, a complete understanding of the atomic nucleus will provide invaluable insights into the fundamental laws of nature and pave the way for future technological innovations. Continued investigation into the dense center of the atom ensures that our understanding of the universe at its most fundamental level will continue to deepen.







.jpg)