The Major Portion Of An Atom's Mass Consists Of
For over a century, scientists have known that atoms, the fundamental building blocks of matter, are not indivisible. They consist of even smaller particles: protons, neutrons, and electrons. While the properties of these subatomic particles are well established, a deeper understanding of how their interactions contribute to the overall mass of an atom continues to fascinate and challenge physicists.
This article explores the crucial role of protons and, particularly, neutrons in contributing to the mass of an atom, highlighting the intricate interplay of fundamental forces within the atomic nucleus. It examines how the vast majority of an atom's mass resides within its nucleus, a densely packed region containing protons and neutrons, collectively known as nucleons.
The Atomic Nucleus: A Dense Core
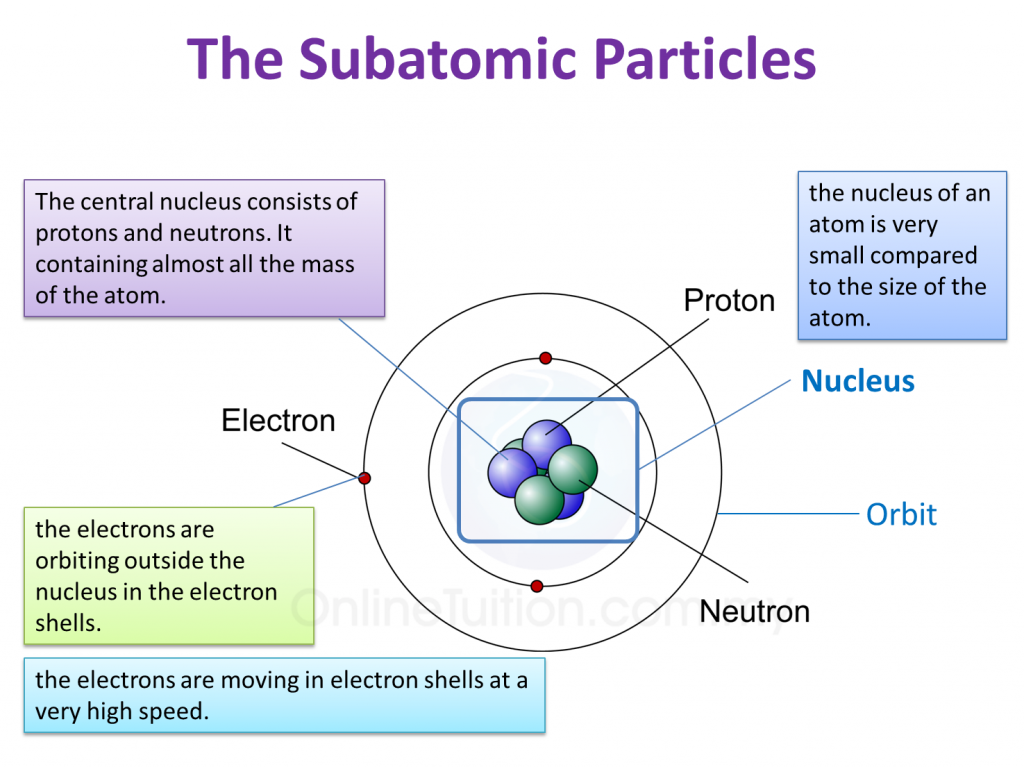
At the heart of every atom lies the nucleus. It's a remarkably compact structure compared to the overall size of the atom. The nucleus houses protons, which carry a positive charge, and neutrons, which are electrically neutral.
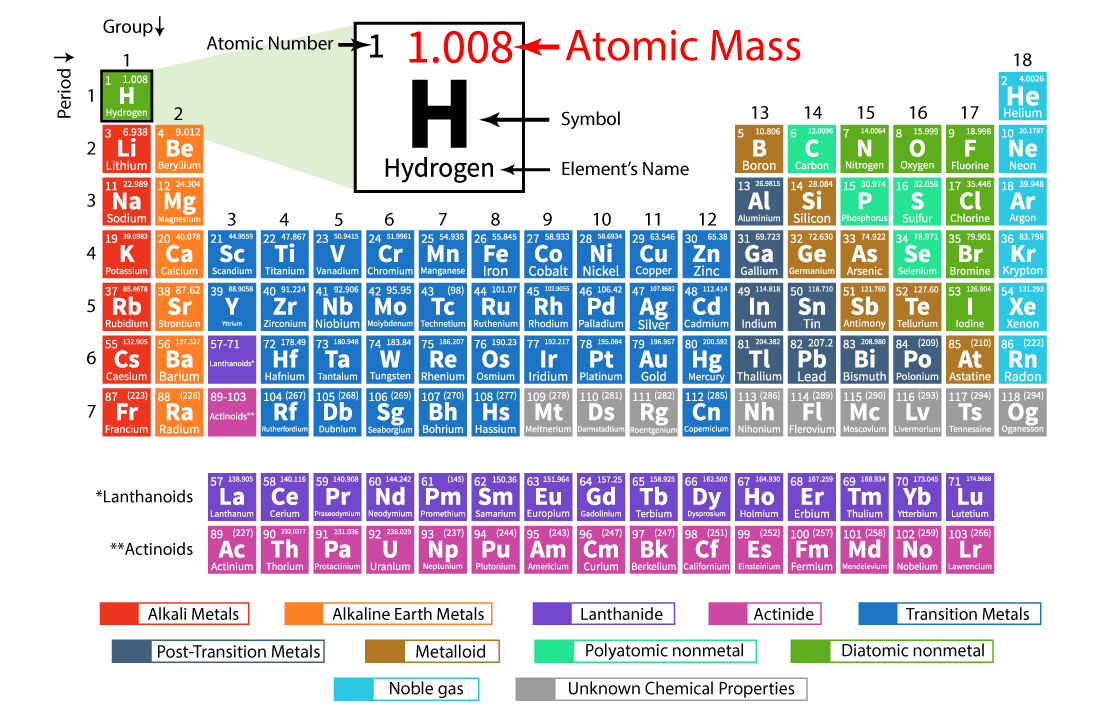
The number of protons determines the element to which the atom belongs. This number is known as the atomic number. The number of neutrons, however, can vary, leading to different isotopes of the same element.
Protons and Neutrons: The Heavyweights
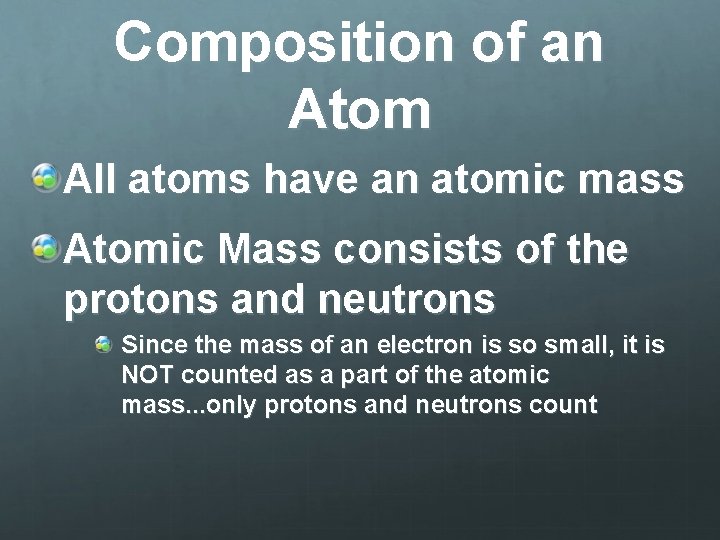
Compared to electrons, protons and neutrons are significantly heavier. A proton is approximately 1,836 times more massive than an electron. A neutron is slightly heavier than a proton, but the difference is relatively small.
Due to their greater mass, protons and neutrons account for approximately 99.9% of an atom's mass. The electrons, although crucial for chemical bonding and reactivity, contribute a negligible amount to the overall mass.
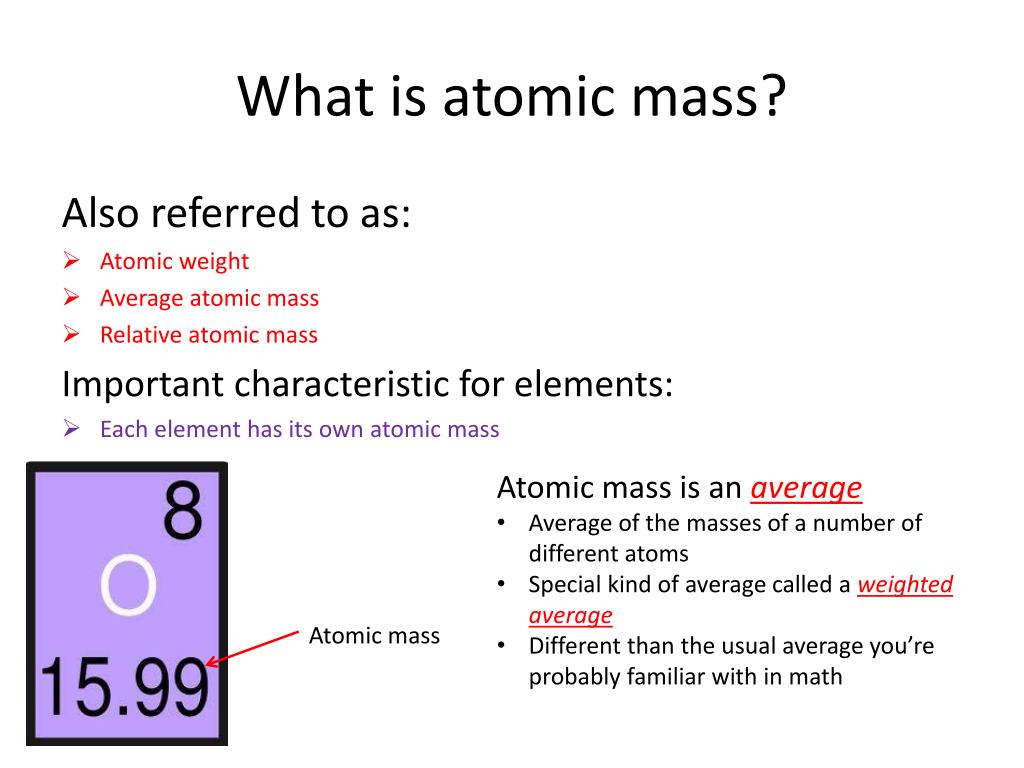
The mass number of an atom represents the total number of protons and neutrons in its nucleus. This number provides a close approximation of the atom's mass in atomic mass units (amu).
The Strong Nuclear Force: Binding the Nucleus
Given that protons are positively charged, one might expect the nucleus to fly apart due to electrostatic repulsion. However, this doesn't happen because of the strong nuclear force. This force is the strongest of the four fundamental forces of nature, and it acts over very short distances within the nucleus.
The strong nuclear force binds protons and neutrons together, overcoming the electrostatic repulsion between protons. It is mediated by particles called gluons, which constantly exchange between the nucleons.
Without the strong nuclear force, the nucleus would be unstable, and atoms as we know them would not exist. This force is crucial for the formation of elements and the stability of matter.
The Role of Neutrons: Nuclear Stability
Neutrons play a crucial role in stabilizing the nucleus, particularly in heavier atoms. As the number of protons increases, the electrostatic repulsion also increases. More neutrons are needed to dilute the positive charge and provide sufficient strong force to hold the nucleus together.
The ratio of neutrons to protons in stable nuclei increases with atomic number. This demonstrates the increasing importance of neutrons in maintaining nuclear stability in heavier elements.
Isotopes with an unstable neutron-to-proton ratio are radioactive. They undergo nuclear decay to achieve a more stable configuration.
Beyond Individual Particles: Mass Defect and Binding Energy
The mass of an atom is not simply the sum of the masses of its individual protons, neutrons, and electrons. There's a slight difference, known as the mass defect.
This mass defect arises from the energy that binds the nucleons together. This energy is known as the binding energy. According to Einstein's famous equation, E=mc², this binding energy corresponds to a small amount of mass.
The greater the binding energy per nucleon, the more stable the nucleus. Iron-56, for instance, has a very high binding energy per nucleon, making it one of the most stable nuclei.
"The strong nuclear force is one of the fundamental forces of nature and is responsible for holding the nucleus together." - Dr. Eleanor Vance, Nuclear Physicist, MIT
Implications and Future Research
Understanding the composition and structure of the atomic nucleus is fundamental to numerous scientific disciplines. This knowledge is crucial for nuclear energy, nuclear medicine, and materials science.
Researchers continue to probe the nucleus to gain a deeper understanding of the strong nuclear force and the behavior of nuclear matter under extreme conditions. Experiments at facilities like CERN's Large Hadron Collider provide valuable insights into the fundamental forces governing the universe.
The exploration of exotic nuclei, with unusual neutron-to-proton ratios, is also an active area of research. It helps scientists refine their models of nuclear structure and stability.
In conclusion, while electrons play a critical role in an atom's chemical properties, the vast majority of an atom's mass is concentrated in its nucleus, primarily contributed by protons and neutrons held together by the strong nuclear force. Further investigation in this domain could unlock new possibilities in various fields.












.jpg)


