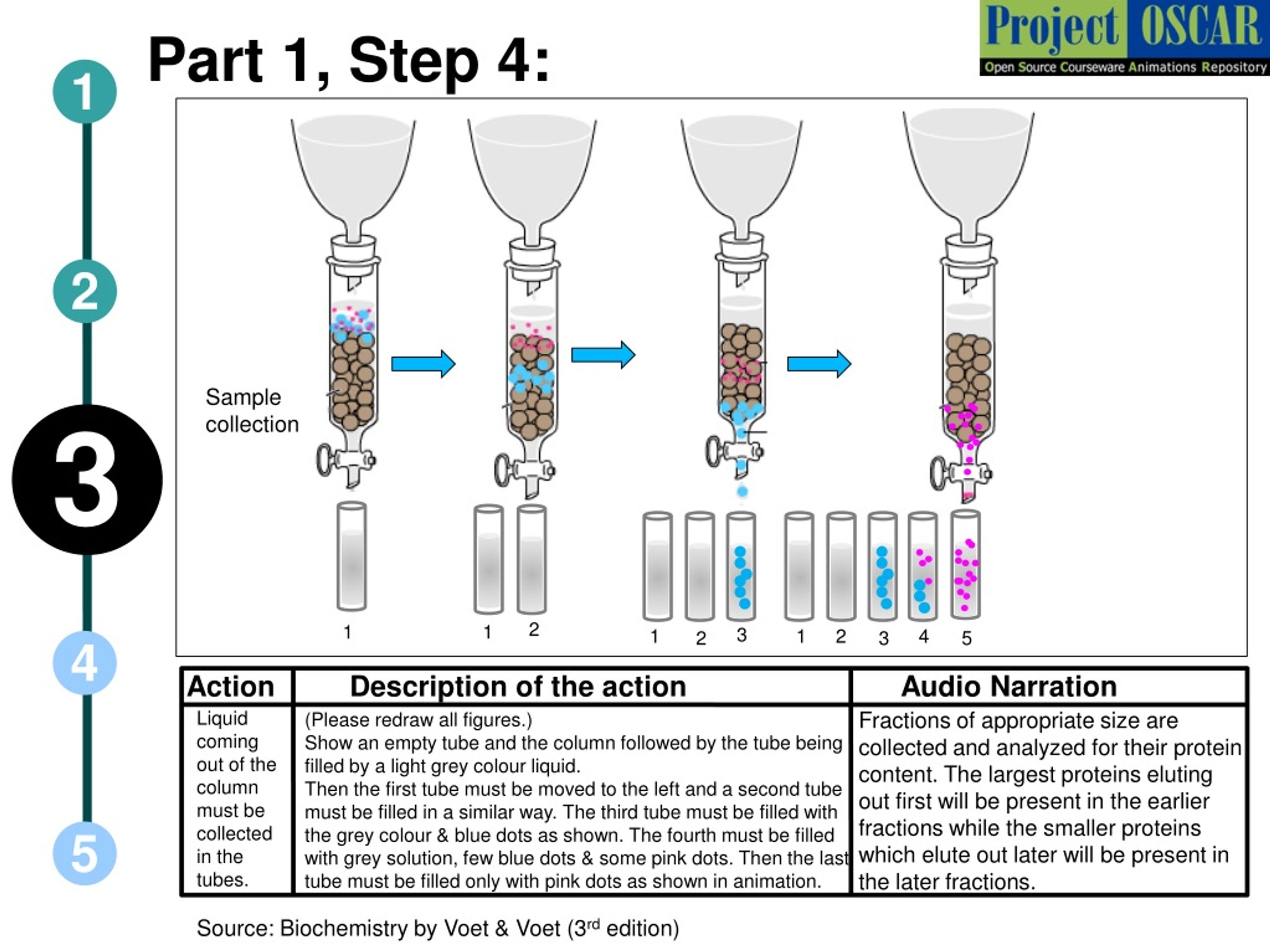
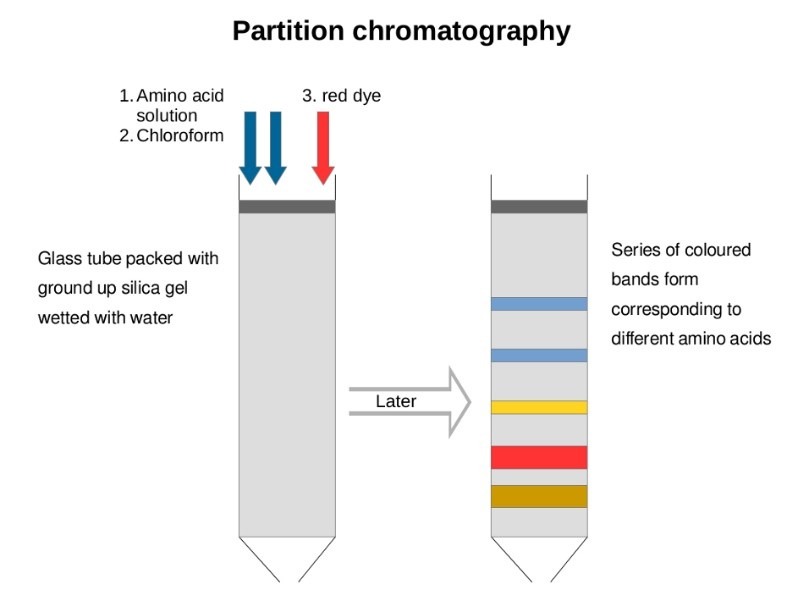
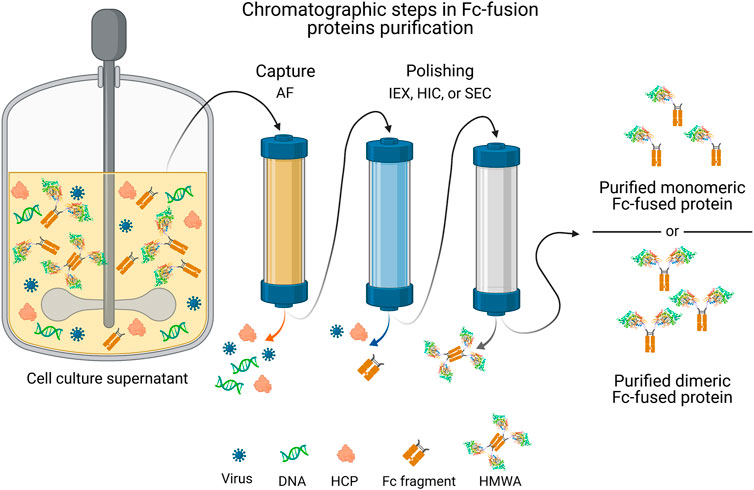
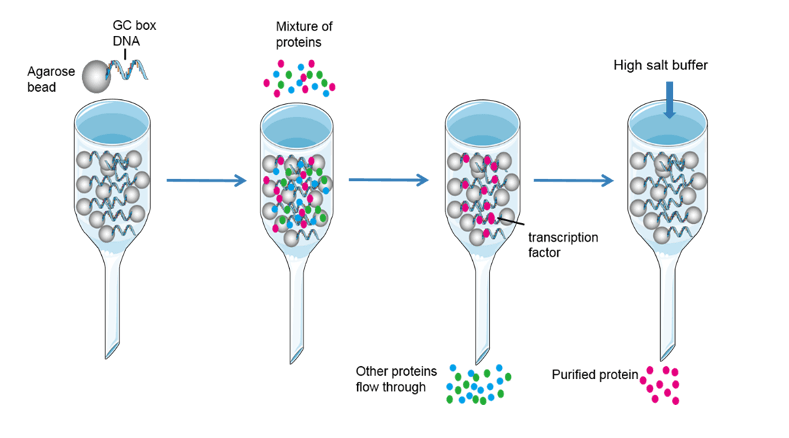
The Second Purification Step Is Which Type Of Chromatographic Separation
The biopharmaceutical industry, a sector dedicated to developing life-saving therapeutics, faces an ongoing challenge: ensuring drug purity and efficacy. This pursuit hinges heavily on meticulously refining complex biological molecules, often proteins, through multiple purification steps. The selection of the optimal chromatographic method for each step is critical, particularly the second, a stage often responsible for removing closely related impurities that eluded the initial capture step. The industry is actively debating and evolving its approach to this vital purification phase.
At the heart of this discourse lies the question: what type of chromatographic separation is best suited for the second purification step in biopharmaceutical manufacturing? This seemingly simple question unravels a complex interplay of factors, including the nature of the target protein, the characteristics of the impurities, and the desired level of purity. Selecting the right chromatography technique at this stage can significantly impact product yield, overall process efficiency, and ultimately, the cost and accessibility of life-saving medications.
Understanding the Landscape of Chromatography
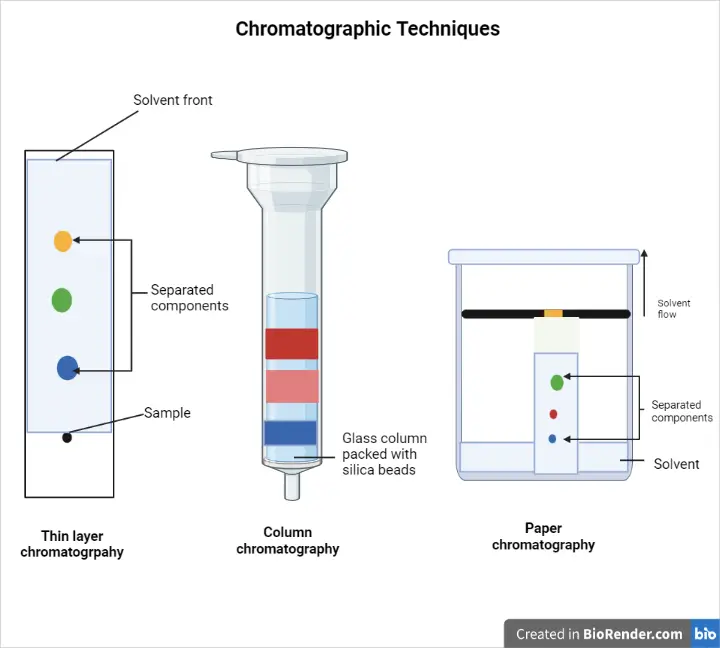
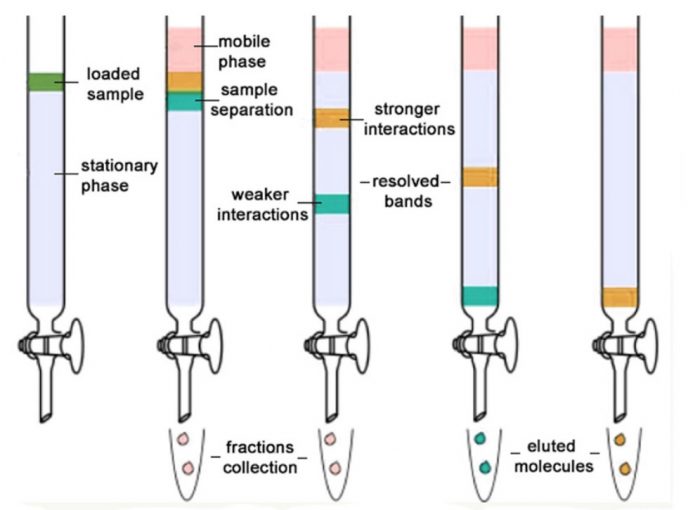
Chromatography, at its core, is a separation technique that relies on the differential distribution of molecules between a stationary phase and a mobile phase. Several types of chromatography are commonly employed in biopharmaceutical purification, each exploiting different physicochemical properties to achieve separation.
Common Chromatography Techniques
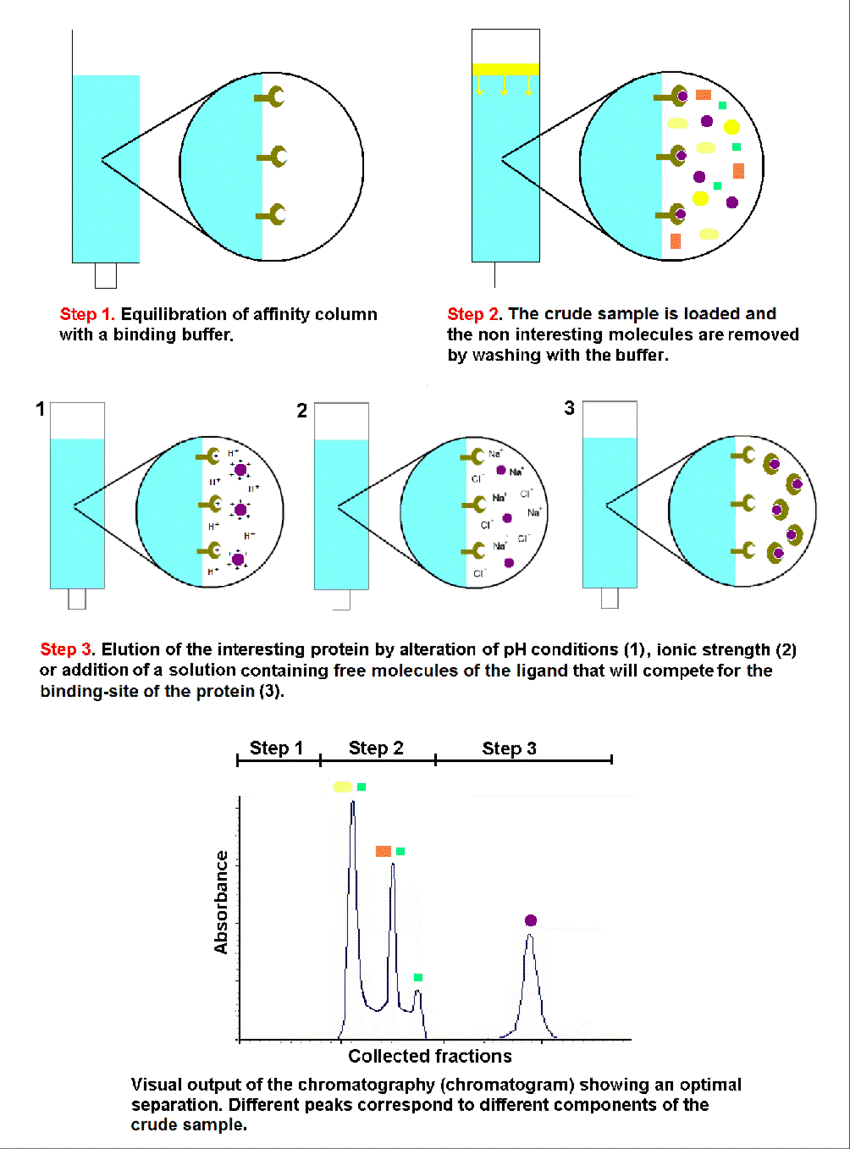
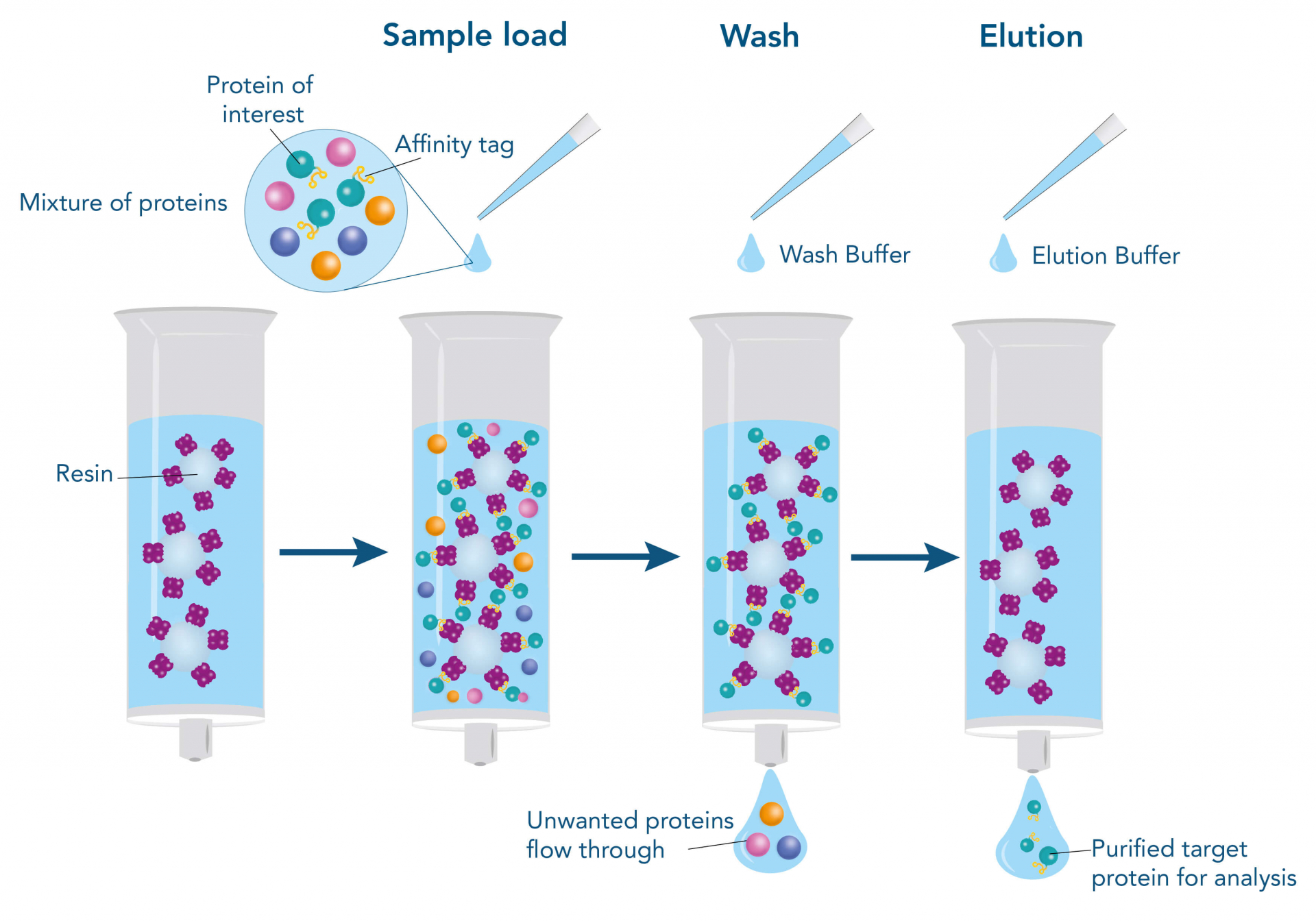
Affinity Chromatography is a highly selective method that utilizes a specific binding interaction between a ligand immobilized on a stationary phase and the target protein. It's often used as a first step for capture, but can also be employed for polishing.
Ion Exchange Chromatography (IEX) separates molecules based on their net charge. Anion exchange chromatography (AEX) binds negatively charged molecules, while cation exchange chromatography (CEX) binds positively charged molecules.
Size Exclusion Chromatography (SEC), also known as gel filtration chromatography, separates molecules based on their size and shape. Larger molecules elute faster than smaller molecules.
Hydrophobic Interaction Chromatography (HIC) separates molecules based on their hydrophobicity. Molecules with greater hydrophobicity bind more strongly to the hydrophobic stationary phase.
Multi-Modal Chromatography combines different interaction mechanisms, such as ion exchange and hydrophobic interactions, providing a broader separation profile.
The Second Step: Refining the Purity Profile
The second purification step, following the initial capture, is crucial for removing impurities with properties similar to the target protein. These impurities can include isoforms, aggregates, host cell proteins (HCPs), and other process-related contaminants.
Industry experts often emphasize that there is no one-size-fits-all solution for the second purification step. The choice of chromatographic method depends heavily on the specific impurities that need to be removed and the characteristics of the target protein. The aim is to resolve the contaminants that remain from the first step.
According to Dr. Emily Carter, a leading bioprocess engineer at BioPharm Solutions, "The second step is where you really start fine-tuning the purity profile. You've already captured your target protein, but now you need to remove those closely related impurities that could compromise safety or efficacy."
The Case for Ion Exchange Chromatography (IEX)
IEX is a frequently favored method for the second purification step. Its ability to resolve molecules based on subtle charge differences makes it effective at removing isoforms, charge variants, and some HCPs.
Dr. Mark Thompson, a chromatography specialist at Global Biotech Innovations, stated, "IEX is a workhorse in biopharmaceutical purification. By carefully selecting the pH and salt concentration, you can selectively elute your target protein while leaving behind the charged impurities."
However, IEX is not without its limitations. It may not be effective at removing impurities that have similar charge properties to the target protein. It also requires careful optimization of buffer conditions to maintain protein stability.
Exploring Alternatives: SEC and HIC
SEC offers an alternative approach, separating molecules based on size. It is particularly useful for removing aggregates and fragments, which can negatively impact product quality.
HIC can be employed to remove hydrophobic impurities, such as certain HCPs or misfolded protein species. Its effectiveness is often maximized by carefully manipulating salt concentrations to optimize the hydrophobic interaction.
The selection between SEC and HIC depends largely on the nature of the remaining impurities after the first step. A thorough understanding of the impurity profile is essential for making an informed decision.
“Ultimately, the goal is to design a robust purification process that consistently delivers a product with the required purity and activity,” explained Dr. Carter.
Multi-Modal Chromatography: A Hybrid Approach
Multi-modal chromatography is gaining traction as a powerful tool for biopharmaceutical purification. By combining different interaction mechanisms, it can provide a broader separation profile and remove a wider range of impurities in a single step.
According to a white paper published by Chromatography Advances Journal, "Multi-modal chromatography offers the potential to simplify purification processes and reduce the number of steps required to achieve the desired purity level."
Despite its potential, multi-modal chromatography is more complex to develop and optimize than traditional chromatography methods. Careful consideration must be given to the selection of the appropriate resin and the optimization of the buffer conditions.
Looking Ahead: The Future of Biopharmaceutical Purification
The field of biopharmaceutical purification is continuously evolving, driven by the need for more efficient, cost-effective, and robust processes. Advances in chromatography resins, process analytics, and modeling are paving the way for improved purification strategies.
The development of novel chromatography techniques and the optimization of existing methods will continue to play a crucial role in ensuring the quality, safety, and accessibility of life-saving biopharmaceutical products. As personalized medicine becomes more prevalent, the ability to rapidly and efficiently purify complex biological molecules will become even more critical.
The debate surrounding the optimal chromatographic method for the second purification step is likely to continue. It emphasizes the importance of a holistic approach to process development, considering the specific characteristics of the target protein, the nature of the impurities, and the overall process objectives. By embracing innovation and continuously seeking improvements, the biopharmaceutical industry can strive to deliver life-saving medications to patients in need.