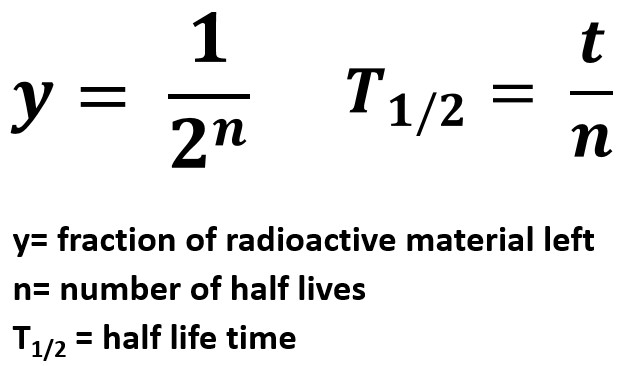The Term Half Life Represents The Time It Takes

Imagine a crisp autumn morning, sunlight filtering through golden leaves, illuminating a Geiger counter gently clicking in a laboratory. The air hums with a quiet energy, a testament to the invisible forces at play as scientists meticulously track the decay of a radioactive isotope. They’re not just observing a scientific process; they're witnessing a fundamental aspect of nature – the concept of half-life in action.
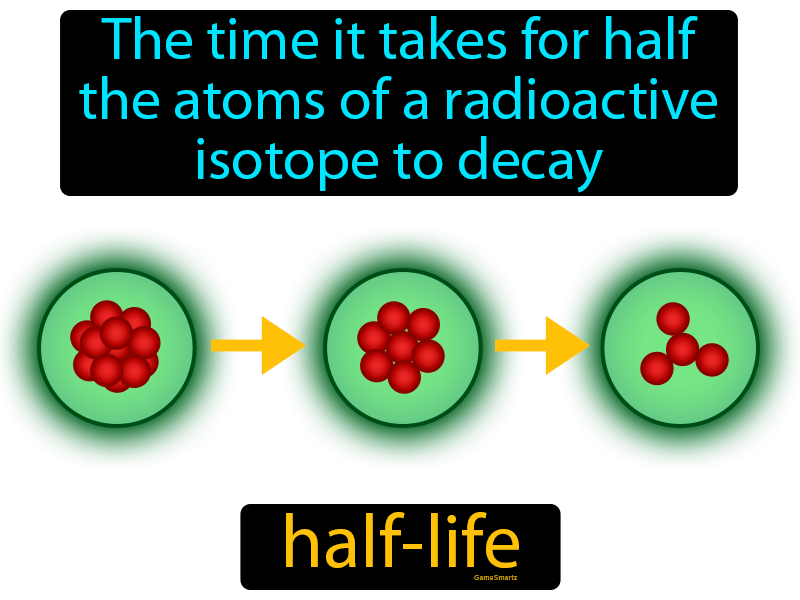
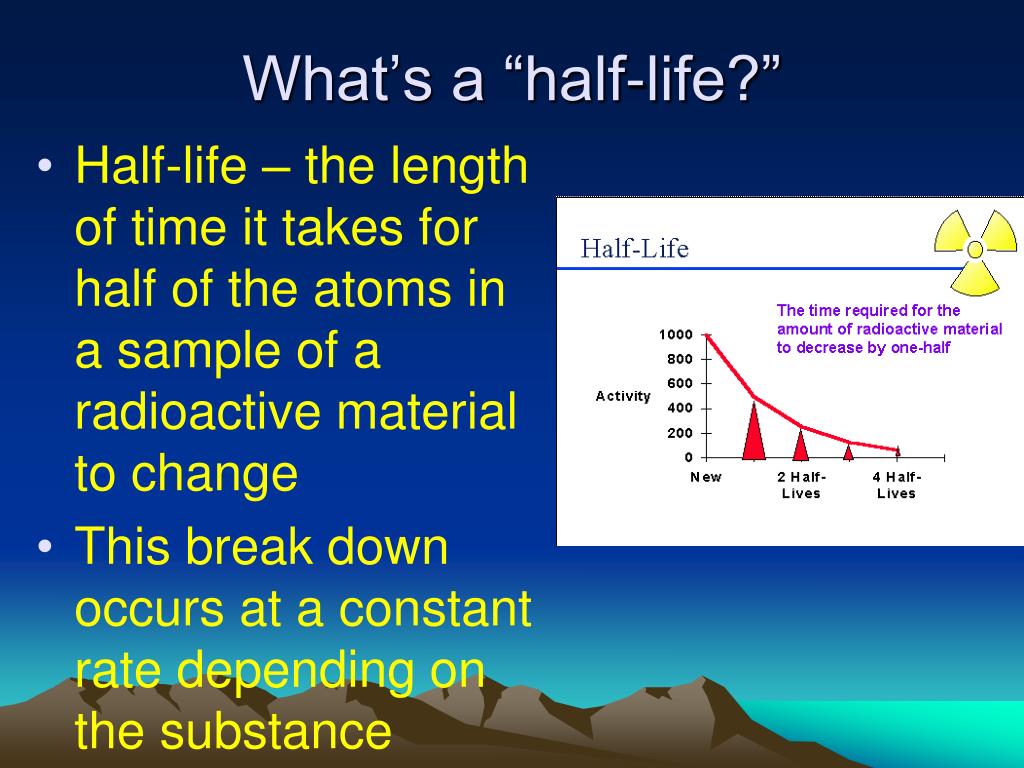
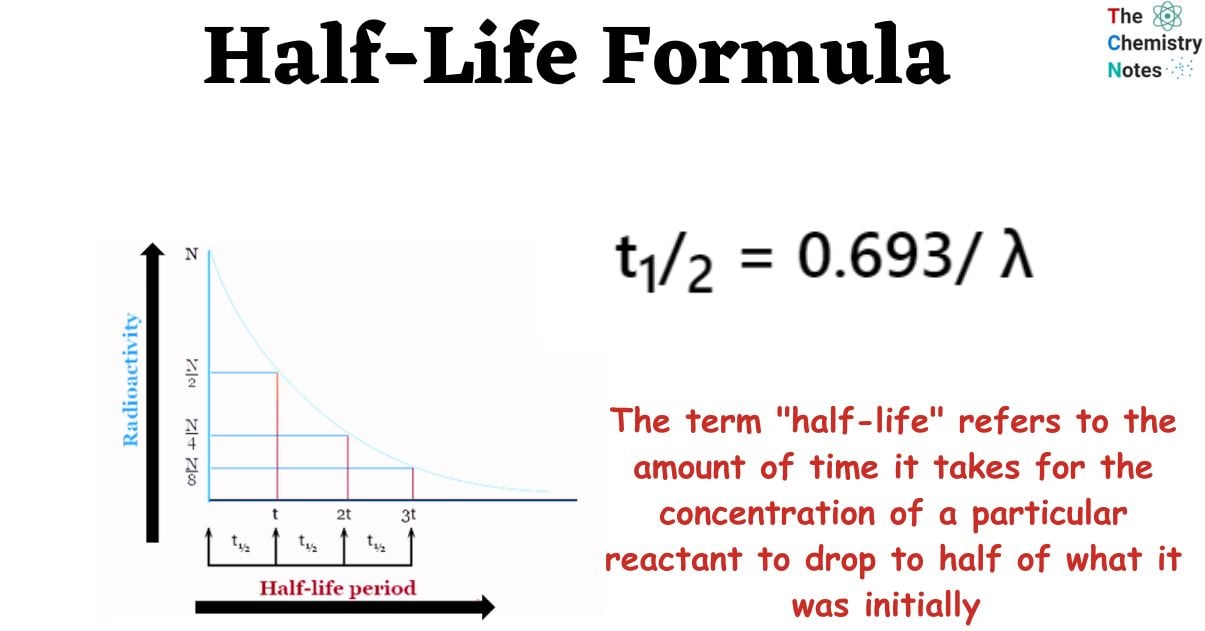
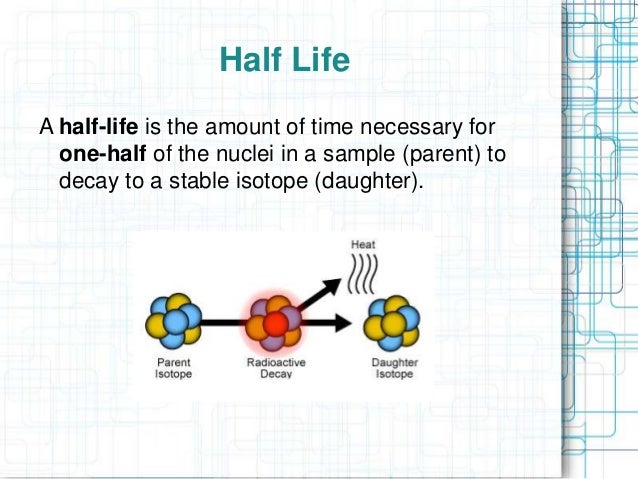
At its core, half-life represents the time it takes for half of a radioactive substance to decay. This deceptively simple definition unveils a concept crucial to fields ranging from medicine and archaeology to environmental science and nuclear energy. Understanding half-life is essential for safe application of many crucial technologies.
A Journey Through Time: Unveiling the Secrets of Decay
The story of half-life begins with the discovery of radioactivity in the late 19th century by scientists like Henri Becquerel and Marie Curie. They noticed that certain elements spontaneously emitted energy, a phenomenon that defied classical physics. This groundbreaking discovery paved the way for understanding the nature of atomic decay and the concept of half-life.
Early researchers observed that the rate of decay was consistent for any given radioactive isotope, regardless of external conditions like temperature or pressure. This led to the realization that the decay process was governed by the intrinsic properties of the atomic nucleus itself. It wasn't a reaction we could speed up or slow down.
Ernest Rutherford, a pioneering figure in nuclear physics, further elucidated the process by identifying different types of radiation – alpha, beta, and gamma. He and his colleagues also demonstrated that the decay of a radioactive element follows an exponential pattern. This exponential pattern provides the foundation for the definition of half-life.
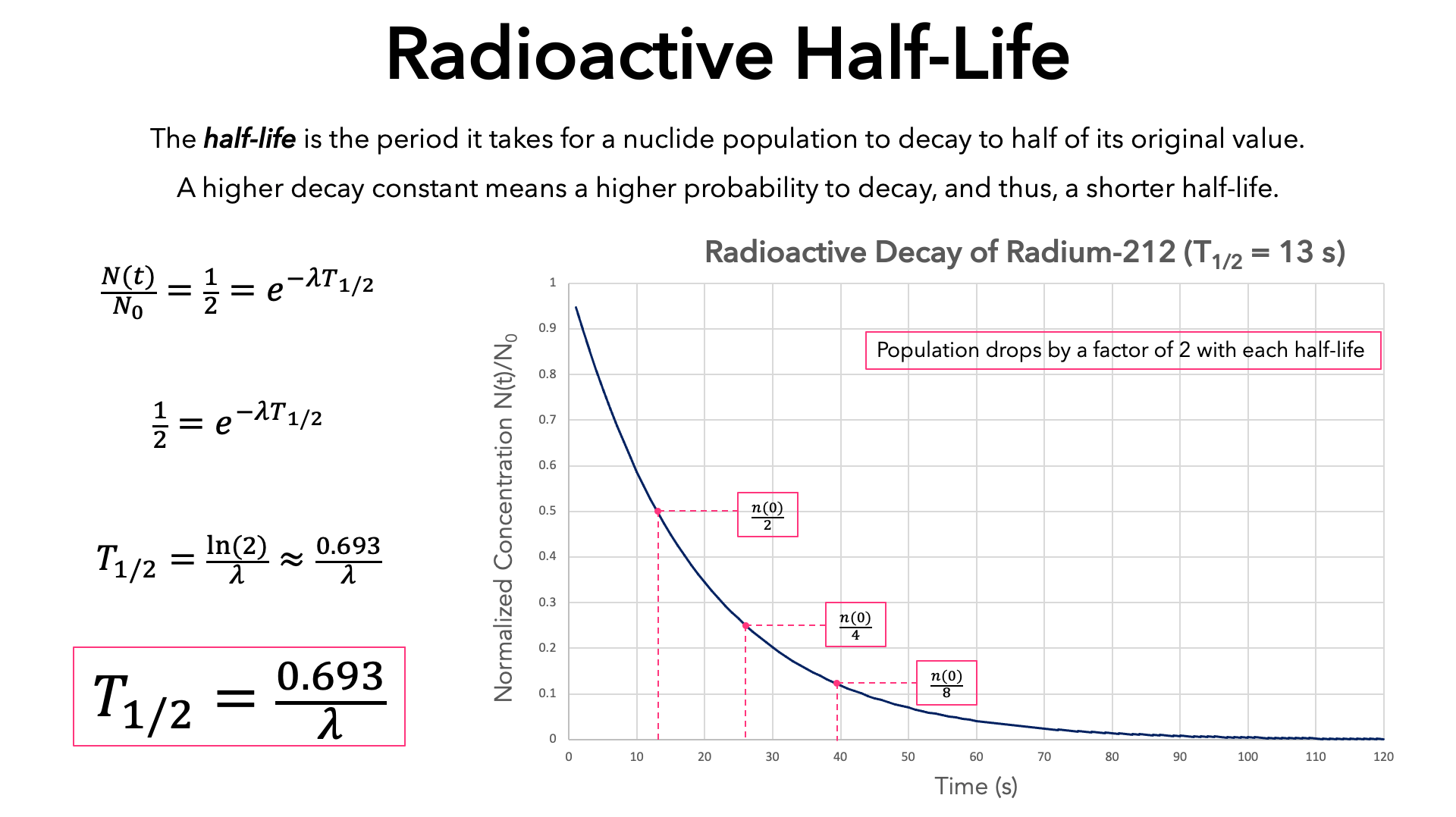
In essence, half-life provides a statistical measure of the time it takes for half of the atoms in a radioactive sample to undergo decay. After one half-life, half of the original atoms will have transformed into a different element or isotope. After two half-lives, only a quarter of the original atoms remain.
This process continues indefinitely, with each subsequent half-life reducing the amount of the original radioactive substance by half. It's important to note that half-life is a probabilistic concept. It doesn't predict when a specific atom will decay, but rather the statistical likelihood of decay within a given timeframe.
The Significance of Half-Life Across Diverse Fields
The concept of half-life is not confined to the realms of physics. It permeates many other disciplines, offering critical insights and enabling innovative applications. Here are some key examples:
Medicine: Diagnostic Imaging and Cancer Treatment
In medicine, radioactive isotopes with short half-lives are frequently used in diagnostic imaging techniques like PET (Positron Emission Tomography) scans. These isotopes are injected into the body and emit radiation that can be detected by specialized cameras, allowing doctors to visualize internal organs and tissues. Technetium-99m, with a half-life of about 6 hours, is a commonly used isotope in medical imaging.
The short half-life of these isotopes minimizes the patient's exposure to radiation. This balance between diagnostic benefit and radiation risk is carefully considered in medical applications. Radioactive isotopes are also used in cancer treatment, where targeted radiation therapy can destroy cancerous cells. The choice of isotope and its half-life are crucial factors in determining the effectiveness and safety of these treatments.
Archaeology: Dating Ancient Artifacts
Half-life plays a pivotal role in radiocarbon dating, a technique used to determine the age of organic materials. Carbon-14, a radioactive isotope of carbon with a half-life of approximately 5,730 years, is used for dating artifacts up to around 50,000 years old. By measuring the amount of Carbon-14 remaining in a sample, archaeologists can estimate when the organism died and provide a timeline for our past.
The ratio of Carbon-14 to stable Carbon-12 in the atmosphere is relatively constant. Living organisms constantly replenish their Carbon-14 supply by absorbing carbon from the environment. However, once an organism dies, it no longer absorbs Carbon-14, and the existing Carbon-14 begins to decay.
By comparing the Carbon-14 content of an artifact to the atmospheric level, scientists can calculate the time elapsed since the organism's death. Radiocarbon dating has revolutionized archaeology and provides invaluable insights into human history.
Environmental Science: Tracking Pollutants
The concept of half-life is also relevant in environmental science for assessing the persistence of pollutants in the environment. Some pollutants, such as certain pesticides and industrial chemicals, can persist in the environment for extended periods due to their long half-lives.
Understanding the half-life of these pollutants is crucial for predicting their environmental fate and potential impact on ecosystems and human health. For example, scientists can use half-life data to model the dispersion and degradation of pollutants in soil, water, and air. This informs strategies for remediation and risk management.
Nuclear Energy: Managing Radioactive Waste
In the realm of nuclear energy, half-life is a critical factor in the safe storage and disposal of radioactive waste. Nuclear waste contains a variety of radioactive isotopes with vastly different half-lives, ranging from fractions of a second to billions of years. For instance, Plutonium-239, a major component of nuclear waste, has a half-life of approximately 24,100 years.
The long half-lives of some of these isotopes pose a significant challenge for long-term waste management. Scientists and engineers are developing various strategies for safely storing nuclear waste for the thousands of years needed for the radioactivity to decay to safe levels. These strategies include geological disposal in stable underground formations and advanced reprocessing technologies to reduce the volume and radiotoxicity of the waste.
Beyond the Numbers: The Profound Implications of Decay
The concept of half-life transcends its scientific definition. It offers a profound perspective on the nature of time, change, and the universe itself. The constant, relentless decay of radioactive substances reminds us of the impermanence of all things.
Like a cosmic clock, half-life provides a way to measure the vastness of geological time and the evolution of the universe. From the formation of elements in the hearts of stars to the decay of radioactive isotopes in the earth's crust, half-life governs the processes that have shaped our planet and our existence.
Understanding half-life is not just about grasping a scientific concept. It's about appreciating the delicate balance between stability and change, and the fundamental laws that govern our world. As we continue to explore the mysteries of the universe, the concept of half-life will undoubtedly remain a guiding principle, illuminating the path towards a deeper understanding of ourselves and our place in the cosmos.