Transcatheter Mitral Valve Repair Pipeline Product Market

The heart, a tireless engine, is increasingly susceptible to breakdowns. Mitral regurgitation, a common valvular heart disease, places an enormous burden on individuals and healthcare systems. Innovation in less invasive treatment options offers a beacon of hope for millions suffering from this debilitating condition, driving fierce competition and rapid advancements in the transcatheter mitral valve repair (TMVr) pipeline product market.
The TMVr market is poised for explosive growth. This article will delve into the rapidly evolving landscape of this market, outlining key players, emerging technologies, clinical trial data, market trends, and future challenges. We will examine the driving forces behind this expansion, from an aging population and the limitations of traditional surgery to the relentless pursuit of less invasive and more effective therapies. The article will also explore potential roadblocks to widespread adoption, including cost-effectiveness concerns, long-term durability data, and the need for specialized training.
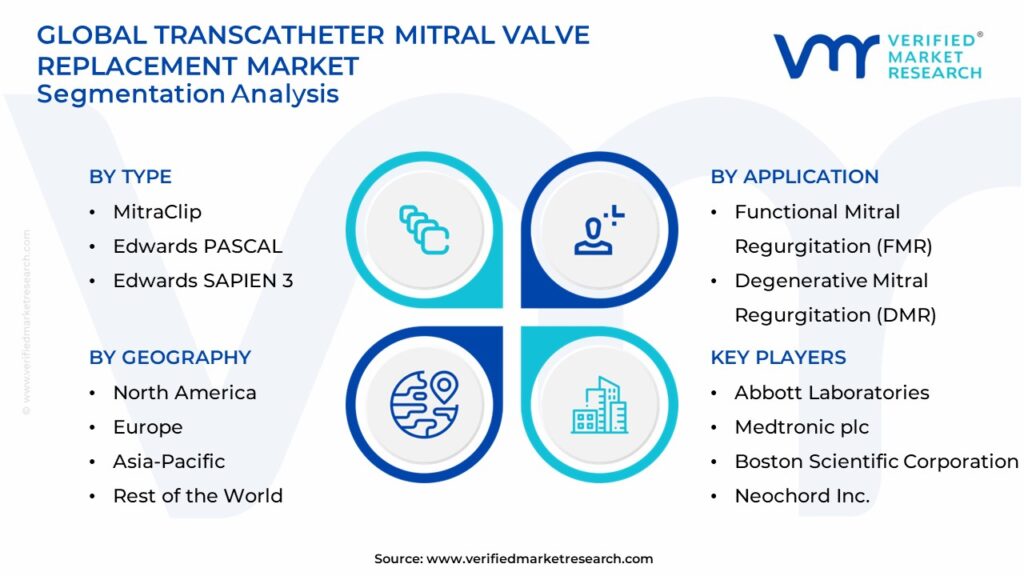
Current Market Landscape and Key Players
The transcatheter mitral valve repair market is currently dominated by a few established players. Abbott, with its MitraClip system, has been a pioneering force and currently holds a significant market share. Edwards Lifesciences, a major player in the transcatheter aortic valve replacement (TAVR) market, is also making significant inroads with its PASCAL system.
These devices employ different mechanisms to address mitral regurgitation. MitraClip utilizes a clip-based approach to approximate the mitral valve leaflets, reducing the backflow of blood. PASCAL, on the other hand, employs a spacer and clasp to achieve a similar effect, offering unique features for challenging anatomies.
Several other companies are developing innovative TMVr technologies. These include companies focusing on direct annuloplasty, leaflet repair, and even transcatheter mitral valve replacement (TMVR), which aims to replace the entire mitral valve without open-heart surgery.
Emerging Technologies and the Pipeline
The TMVr pipeline is rich with promising new technologies. Many companies are developing next-generation clip systems with improved maneuverability and leaflet grasping capabilities. Others are exploring novel annuloplasty approaches, aiming to reshape the mitral valve annulus and restore its natural function.
Direct annuloplasty devices are designed to cinch the mitral valve annulus, reducing its size and improving leaflet coaptation. These devices are typically delivered percutaneously and anchored to the annulus using various fixation mechanisms.
TMVR represents the most ambitious approach. These devices aim to completely replace the diseased mitral valve with a bioprosthetic valve delivered via catheter. While TMVR offers the potential for a more complete and durable solution, it also presents significant technical challenges.
Clinical Trial Data and Efficacy
Clinical trial data plays a crucial role in shaping the TMVr market. The COAPT trial, which studied the MitraClip in patients with heart failure and severe mitral regurgitation, showed significant benefits in terms of heart failure hospitalizations and mortality.
However, the MITRA-FR trial, which studied the MitraClip in patients with functional mitral regurgitation, did not show a significant benefit. These conflicting results highlight the importance of patient selection and the need for careful consideration of individual patient characteristics.
Ongoing clinical trials are evaluating the safety and efficacy of new TMVr technologies. These trials are crucial for demonstrating the value of these devices and securing regulatory approvals.
Market Trends and Growth Drivers
Several factors are driving the growth of the TMVr market. The aging population, with its increased prevalence of valvular heart disease, is a major driver. The desire for less invasive treatment options is also fueling demand.
The limitations of traditional open-heart surgery, particularly in elderly and high-risk patients, have spurred the development of TMVr. Advancements in imaging technologies, such as transesophageal echocardiography (TEE) and cardiac computed tomography (CT), have improved patient selection and procedural guidance.
Increasing awareness among physicians and patients about the availability of TMVr is also contributing to market growth. Improved reimbursement policies and expanded access to TMVr procedures will further accelerate adoption.
Challenges and Roadblocks
Despite its significant potential, the TMVr market faces several challenges. Cost-effectiveness remains a concern, particularly in resource-constrained healthcare systems. Long-term durability data is also needed to assess the long-term performance of TMVr devices.
The need for specialized training and expertise is a barrier to widespread adoption. Optimal patient selection is crucial for achieving successful outcomes. Furthermore, there is concern about the risk of complications, such as bleeding, stroke, and device-related issues.
Regulatory hurdles and lengthy approval processes can also delay the introduction of new TMVr technologies. Addressing these challenges will be crucial for realizing the full potential of the TMVr market.
The Future of TMVr
The future of the transcatheter mitral valve repair market is bright. Continued innovation in device design and procedural techniques will lead to improved outcomes and expanded indications. The development of more durable and effective TMVr technologies will drive further adoption.
Personalized approaches to TMVr, tailored to individual patient anatomy and physiology, will become increasingly important. Integration of artificial intelligence (AI) and machine learning (ML) into TMVr procedures will improve patient selection, procedural planning, and outcomes.
Ultimately, the TMVr market is poised to transform the treatment of mitral regurgitation. These advancements promise to significantly improve the lives of millions of patients suffering from this debilitating condition. The race to innovate and refine these life-saving technologies continues, promising a future where mitral valve disease is managed with greater precision and less invasiveness. This is a market driven by necessity and fueled by innovation, creating a dynamic and ever-evolving landscape.