What Does The Law Of Multiple Proportions Account For

Imagine stepping into a bustling kitchen. Ingredients are scattered, yet a skilled chef knows exactly how much of each is needed to create a perfect dish. Flour, sugar, eggs – combine them in specific ratios, and you get a cake. Change the ratios, and you might end up with something completely different, or even inedible. This culinary dance of proportions mirrors a fundamental principle in chemistry: The Law of Multiple Proportions.
This law, a cornerstone of modern chemistry, essentially explains why elements can combine in different ways to form multiple compounds. It provides a framework for understanding how the weights of elements in these compounds relate to one another, ensuring a consistent and predictable system in the vast landscape of chemical combinations.
A Historical Glimpse: Proust and Dalton
The concept of fixed proportions, a precursor to the Law of Multiple Proportions, gained momentum in the late 18th century. Joseph Proust, a French chemist, championed the Law of Definite Proportions. This law stated that a chemical compound always contains exactly the same proportion of elements by mass, regardless of the size of the sample or the method of preparation. For example, pure water always contains approximately 11.19% hydrogen and 88.81% oxygen by mass.
However, Proust's ideas weren't universally accepted at first. He engaged in debates with other scientists, most notably Claude Louis Berthollet, who believed that elements could combine in any proportion. The eventual acceptance of Proust's Law of Definite Proportions paved the way for further discoveries about how elements combine.
The real breakthrough came with John Dalton, an English chemist and physicist, who formulated the Law of Multiple Proportions in the early 19th century. Dalton meticulously studied the composition of various compounds, carefully measuring the relative weights of elements.
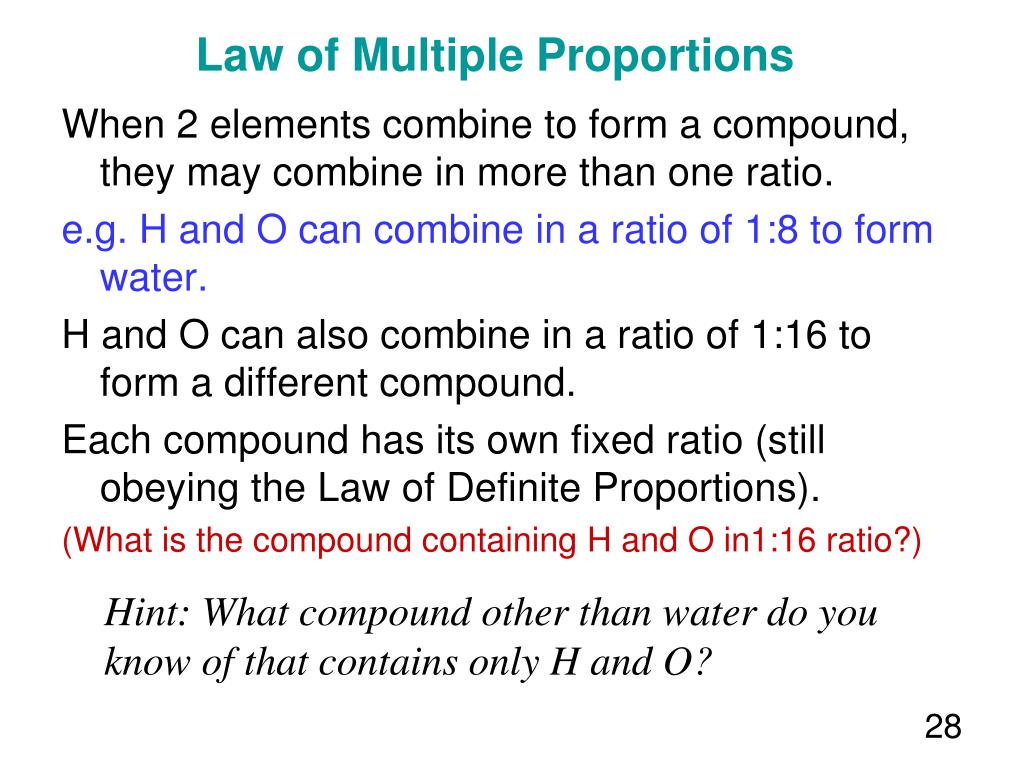
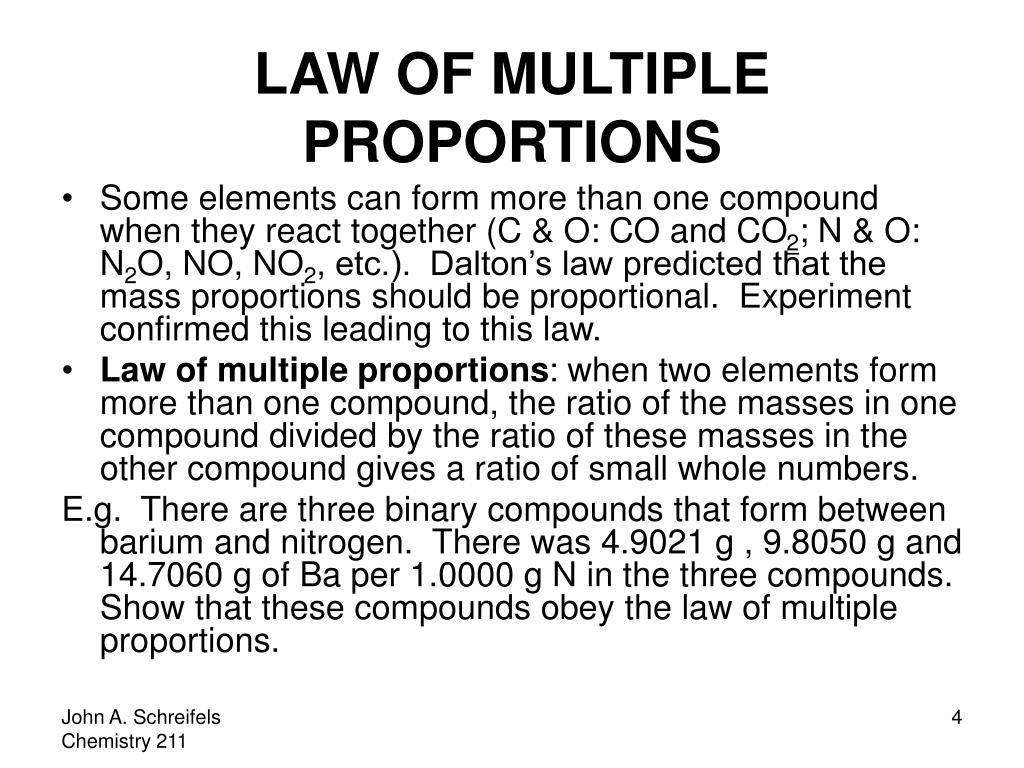
He observed that if two elements form more than one compound, then the ratios of the masses of the second element which combine with a fixed mass of the first element will always be ratios of small whole numbers. This observation provided strong evidence for the existence of atoms and the idea that chemical reactions involve the rearrangement of these atoms.
Decoding Dalton's Law: Simple Ratios
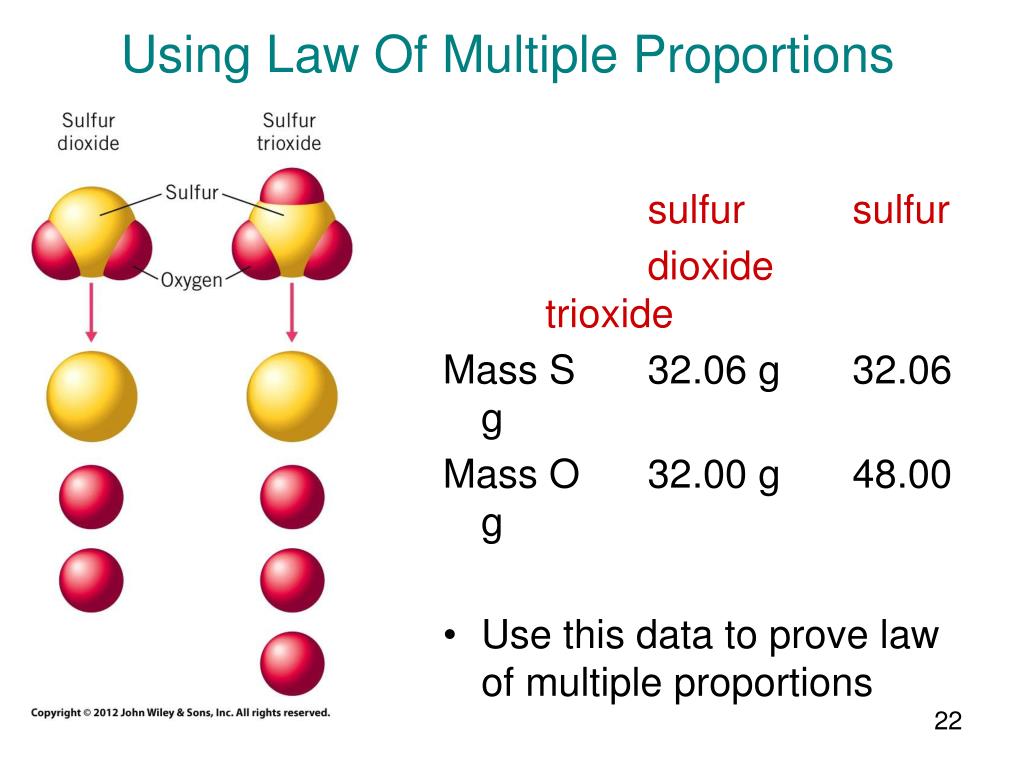
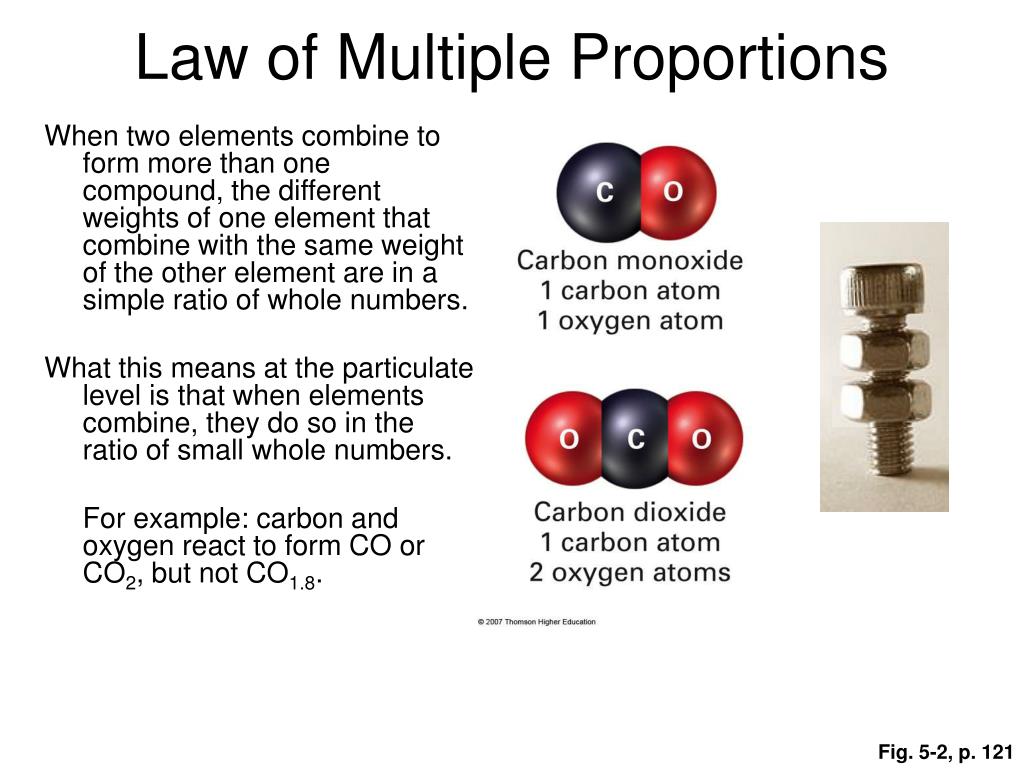
Let's consider carbon and oxygen. These two elements can combine to form two common compounds: carbon monoxide (CO) and carbon dioxide (CO2). In carbon monoxide, 12 grams of carbon combine with 16 grams of oxygen. In carbon dioxide, 12 grams of carbon combine with 32 grams of oxygen.
Now, let's fix the mass of carbon at 12 grams. We see that the ratio of the masses of oxygen that combine with 12 grams of carbon is 16:32, which simplifies to 1:2 – a small whole-number ratio. This elegantly demonstrates the Law of Multiple Proportions in action.
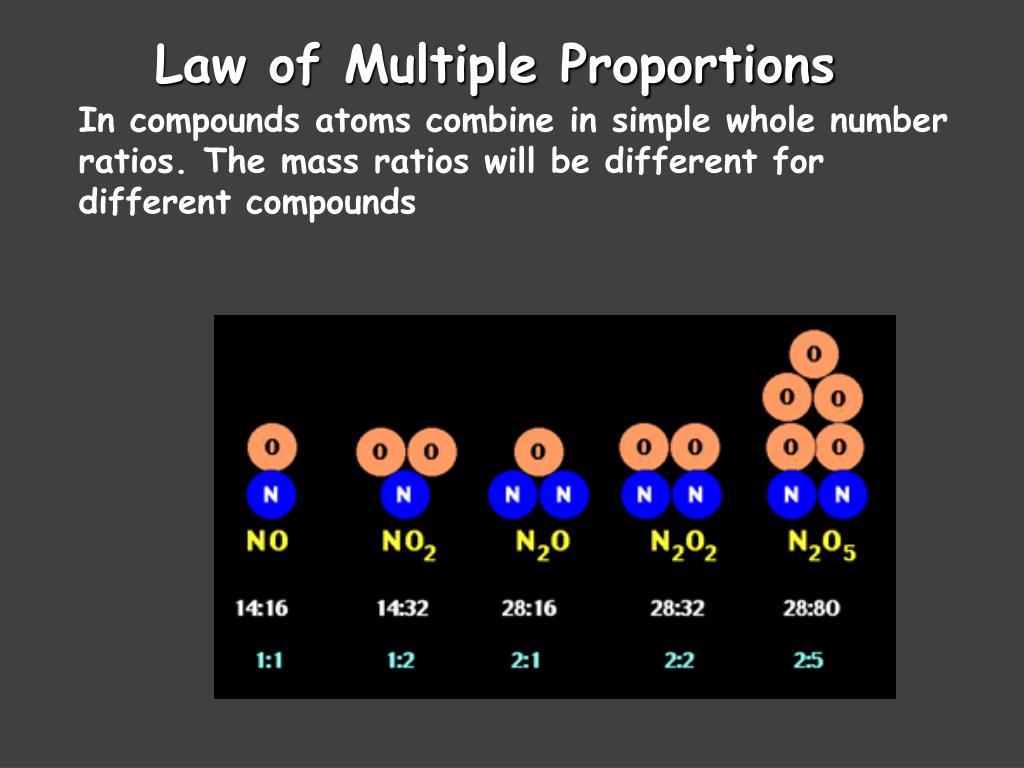
Another common example involves nitrogen and oxygen, which can form several different compounds, including nitrous oxide (N2O), nitric oxide (NO), and nitrogen dioxide (NO2). By fixing the mass of nitrogen and comparing the masses of oxygen, we consistently find small whole-number ratios. This pattern reinforces the fundamental principle that atoms combine in definite, quantifiable ways.
Atomic Theory and the Law of Multiple Proportions
Dalton's Law of Multiple Proportions provided crucial support for his atomic theory. The law suggested that elements combine in fixed, discrete units, which aligned perfectly with the concept of atoms. If matter were continuous, there would be no reason to expect these simple whole-number ratios.
Dalton proposed that each element is composed of identical atoms, and that these atoms can combine with each other in simple whole-number ratios to form compounds. This groundbreaking idea revolutionized chemistry, providing a new framework for understanding the nature of matter and chemical reactions.
His atomic theory, supported by the Law of Multiple Proportions, helped to explain many observed chemical phenomena, such as the conservation of mass during chemical reactions. It also paved the way for further advancements in understanding the structure of atoms and the nature of chemical bonding.
Significance and Modern Applications
The Law of Multiple Proportions is far more than just a historical curiosity. It laid the foundation for modern stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. Stoichiometry allows chemists to predict the amounts of substances needed for a reaction and the amounts of products that will be formed.
This law is vital in chemical analysis, where the composition of unknown substances must be determined. By carefully measuring the masses of elements in a compound, chemists can deduce its chemical formula and identify its components.
The law also helps us understand the formation of complex molecules, like polymers and pharmaceuticals. These complex molecules are built from repeating units, and understanding the proportions of the different elements is crucial for synthesizing and characterizing them.
Beyond the Textbook
The Law of Multiple Proportions also has implications beyond the laboratory. For example, it plays a role in environmental science. Understanding the ratios of different pollutants in the atmosphere, like nitrogen oxides, helps scientists to develop strategies for reducing air pollution.
In materials science, the law is crucial for designing new materials with specific properties. The composition of alloys, ceramics, and composites must be carefully controlled to achieve desired characteristics like strength, conductivity, and corrosion resistance.
Even in the food industry, the Law of Multiple Proportions is indirectly applicable. Understanding the ratios of different nutrients, like carbohydrates, proteins, and fats, helps to develop balanced diets and optimize food processing techniques.
A Lasting Legacy
The Law of Multiple Proportions might seem like a simple concept, but its impact on chemistry has been profound. It provides a fundamental understanding of how elements combine to form compounds, laying the foundation for countless discoveries and innovations.
From the development of new medicines to the creation of advanced materials, this law continues to shape our understanding of the world around us. It reminds us that even the most complex phenomena can be understood by identifying the underlying patterns and principles.
So, the next time you're in the kitchen, remember the chef carefully measuring ingredients. Think of John Dalton and his groundbreaking law, and appreciate the elegant order that governs the chemical world. The Law of Multiple Proportions is a testament to the power of observation, experimentation, and the human desire to understand the fundamental laws of nature.










.jpg)