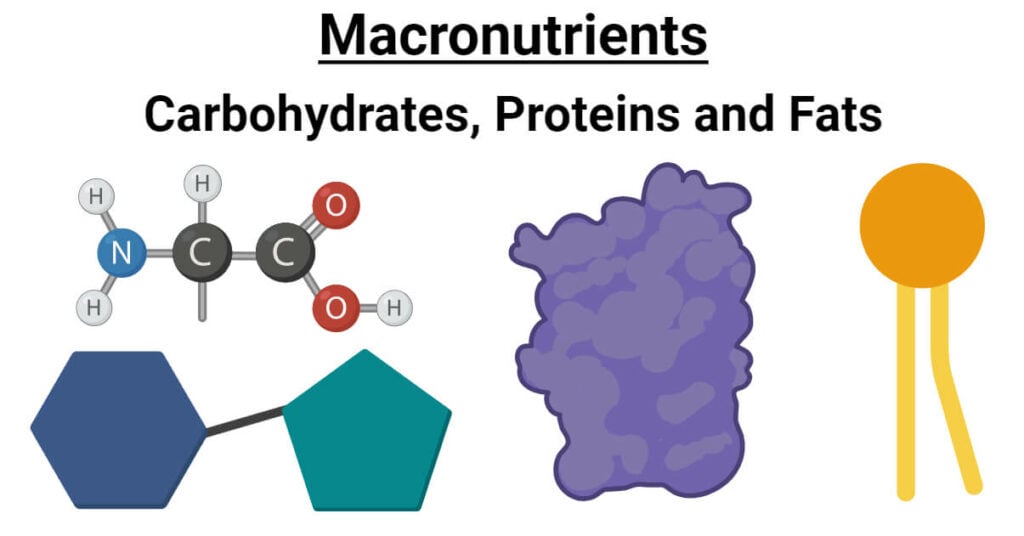
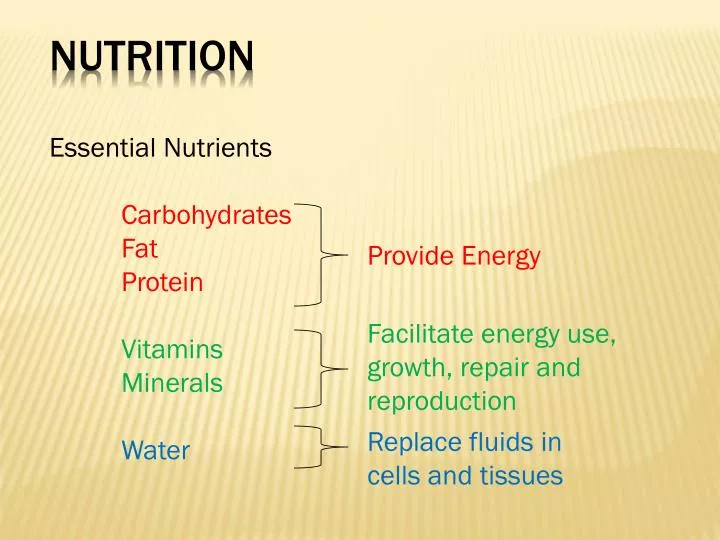
What Element Makes Protein Different From Carbohydrate And Fat
The building blocks of life, each playing a unique role in the intricate machinery of our bodies. Carbohydrates fuel our immediate energy needs, fats provide long-term storage and insulation, but one key element distinguishes proteins and grants them their unparalleled versatility. It's not just the arrangement of carbon, hydrogen, and oxygen, but the presence of a single, critical atom that unlocks a world of functionality.
This article delves into the heart of biochemistry to uncover the element that sets proteins apart: Nitrogen. This seemingly small addition to the molecular structure has profound consequences for the role proteins play in our bodies, their diverse functionalities, and even their dietary impact. We will explore how nitrogen enables proteins to build and repair tissues, create enzymes and hormones, and perform countless other essential tasks that carbohydrates and fats simply cannot accomplish.
The Core Elements: Carbon, Hydrogen, and Oxygen
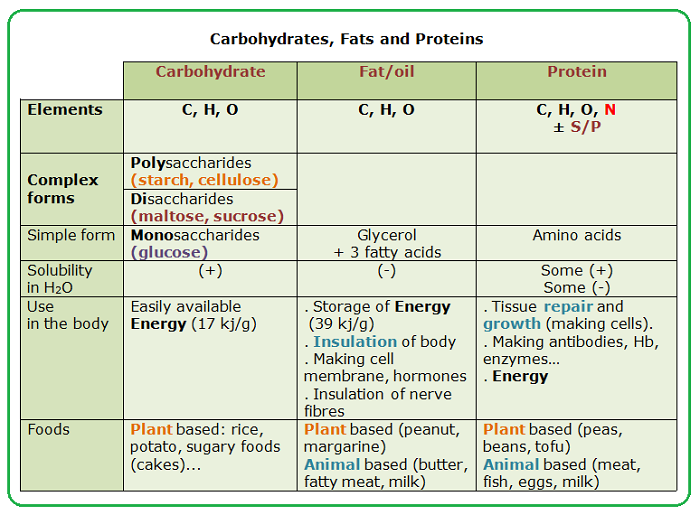
At the fundamental level, carbohydrates, fats (lipids), and proteins share a common trio of elements: carbon, hydrogen, and oxygen. These elements form the backbone of their respective molecular structures, giving rise to a range of different arrangements and ratios.
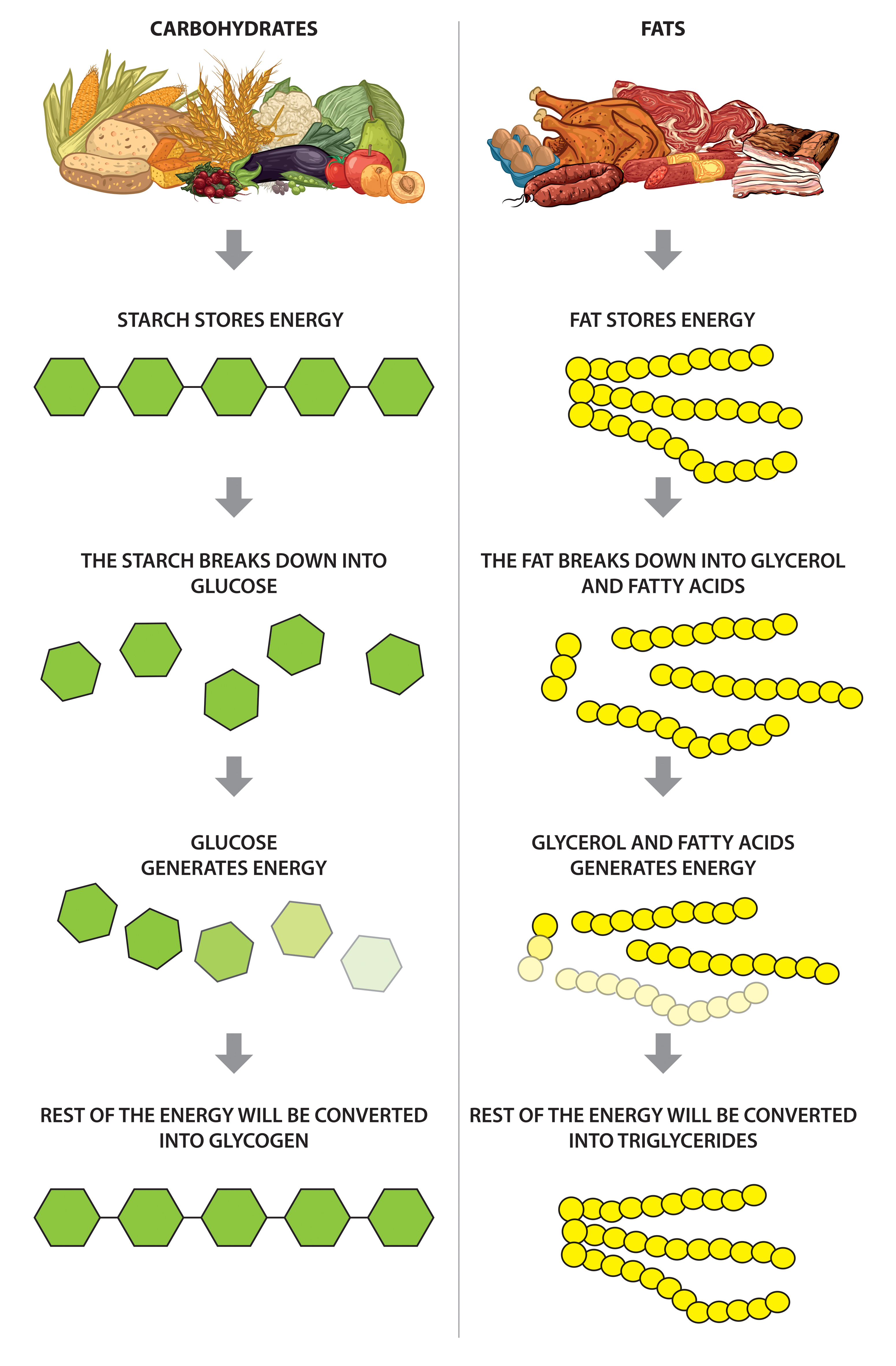
Carbohydrates, often represented by the formula (CH2O)n, are primarily composed of sugars and starches. They serve as a readily available source of energy for the body.
Fats, on the other hand, are composed of glycerol and fatty acids, packed with carbon-hydrogen bonds, storing more energy per gram than carbohydrates. These elements play a crucial role in energy storage, insulation, and hormone production.
The Defining Difference: Nitrogen's Role
Proteins contain carbon, hydrogen, and oxygen just like fats and carbohydrates, but their distinguishing characteristic is the presence of nitrogen. This seemingly small addition has immense consequences.
Nitrogen is incorporated into amino acids, the building blocks of proteins. Each amino acid possesses an amino group (NH2) containing nitrogen, which is crucial for forming peptide bonds and creating complex protein structures.
Without nitrogen, amino acids would be unable to link together, and proteins couldn't form. This would eliminate all of the biological functions that depend on these molecules.
Amino Acids: The Building Blocks
Proteins are polymers assembled from smaller units called amino acids. There are 20 common amino acids, each with a unique side chain (R-group) that dictates its properties and interaction with other molecules.
The amino group (NH2) of one amino acid reacts with the carboxyl group (COOH) of another, forming a peptide bond. This reaction releases a molecule of water and creates a chain of amino acids known as a polypeptide.
The sequence of amino acids dictates the protein’s three-dimensional structure and ultimately, its function. The precise order of amino acids, influenced by DNA, enables proteins to adopt specific shapes and interact with other molecules in a precise way.
Protein Functions: Beyond Energy
The presence of nitrogen allows proteins to perform a vast array of functions far beyond simply providing energy. Proteins are the workhorses of the cell, performing tasks ranging from catalyzing biochemical reactions to transporting molecules and providing structural support.
Enzymes, biological catalysts that speed up chemical reactions, are proteins. Antibodies, part of the immune system that defend against foreign invaders, are also proteins.
"Proteins are directly involved in most of the body's functions,"as stated by the National Institutes of Health (NIH) in a recent publication.
Hormones, such as insulin, and structural components like collagen and keratin, are all proteins, showcasing the diversity of their functions.
Dietary Implications and Protein Metabolism
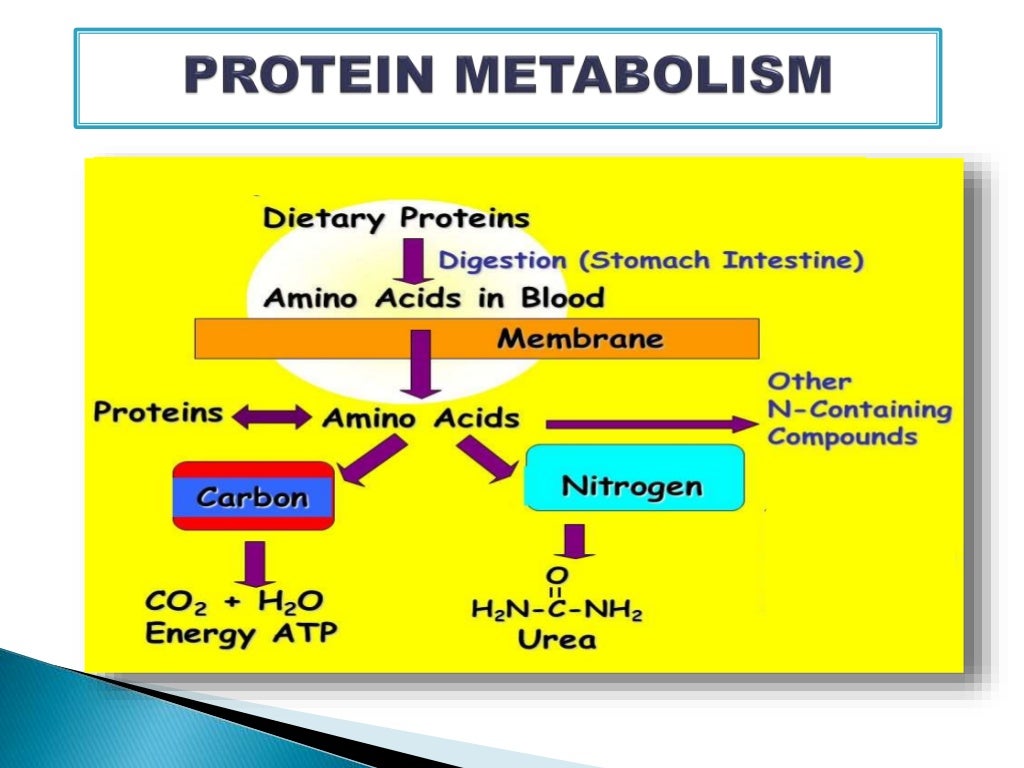
Because the human body cannot store nitrogen effectively, a regular intake of protein is necessary to replenish the amino acid pool. Dietary protein is broken down into amino acids, which are then used to synthesize new proteins or other nitrogen-containing compounds.
Excess amino acids cannot be stored directly and are deaminated. The nitrogen is converted to urea and excreted in urine, highlighting the body's efficient waste-removal process.
Insufficient protein intake can lead to muscle wasting, impaired immune function, and other health problems. This is especially important to consider for vegetarians, vegans, and people with certain medical conditions who may require special attention to ensure adequate protein intake.
Future Research and Protein Engineering
The understanding of the crucial role of nitrogen in protein structure and function continues to drive research in various fields. Scientists are exploring protein engineering techniques to design novel proteins with specific properties and applications.
These engineered proteins have the potential to revolutionize medicine, biotechnology, and materials science. Modifying the amino acid sequence of proteins, and understanding how this impacts the nitrogen bonding and the subsequent protein structure and function can unlock new treatments for disease and create new materials with unparalleled properties.
Further research into nitrogen metabolism and protein synthesis can lead to a deeper understanding of human health and disease. As we continue to unravel the mysteries of this vital element, new possibilities for improving human life will undoubtedly emerge.