What Is The Current Model Of An Atom Called

Imagine peering into a realm far smaller than anything visible to the naked eye – a world where particles dance with probabilistic grace and energy hums in discrete packets. This isn't science fiction; it's the atomic level, the fundamental building block of everything around us. For centuries, scientists have strived to understand the atom's structure, piecing together clues like detectives solving a cosmic puzzle.
At the heart of it all lies a quest to understand: What exactly is the current model of the atom called, and how did we get here?
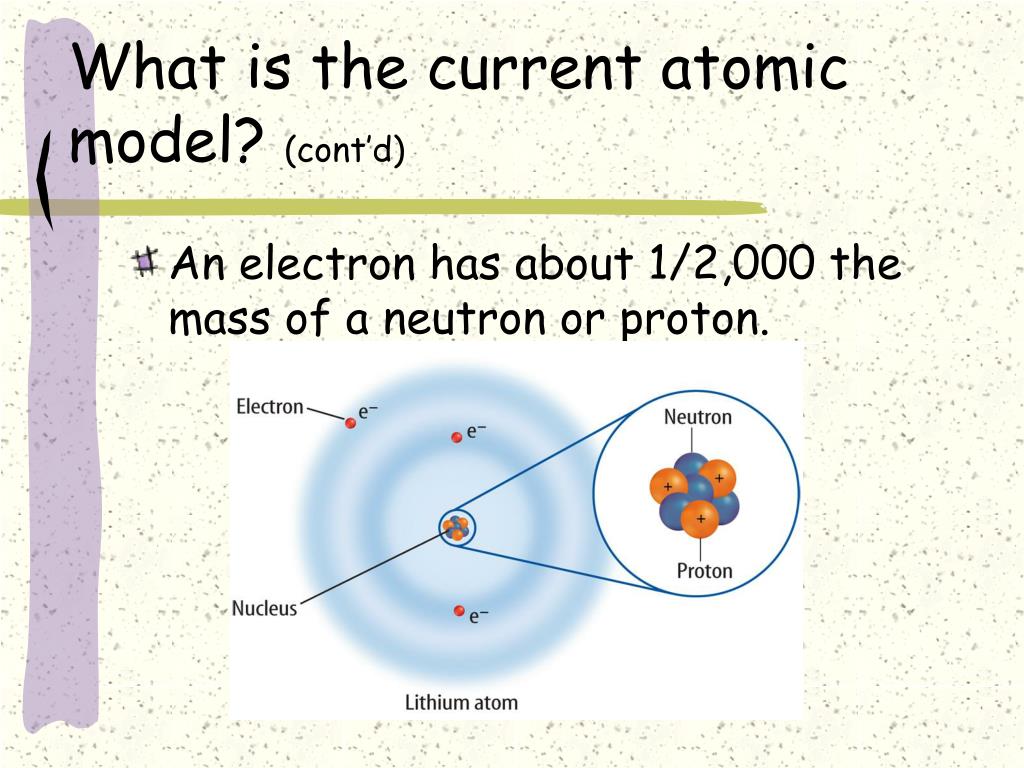
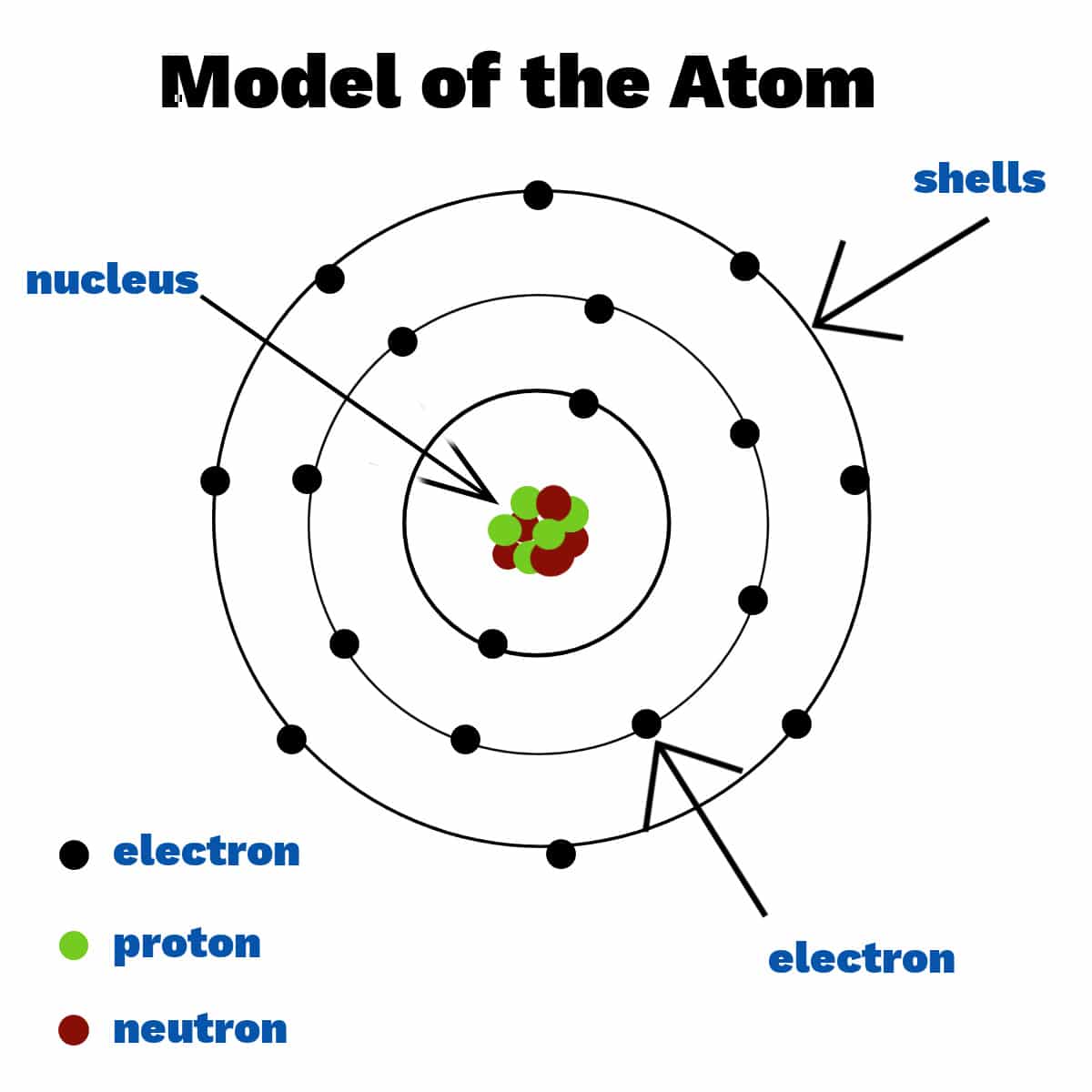
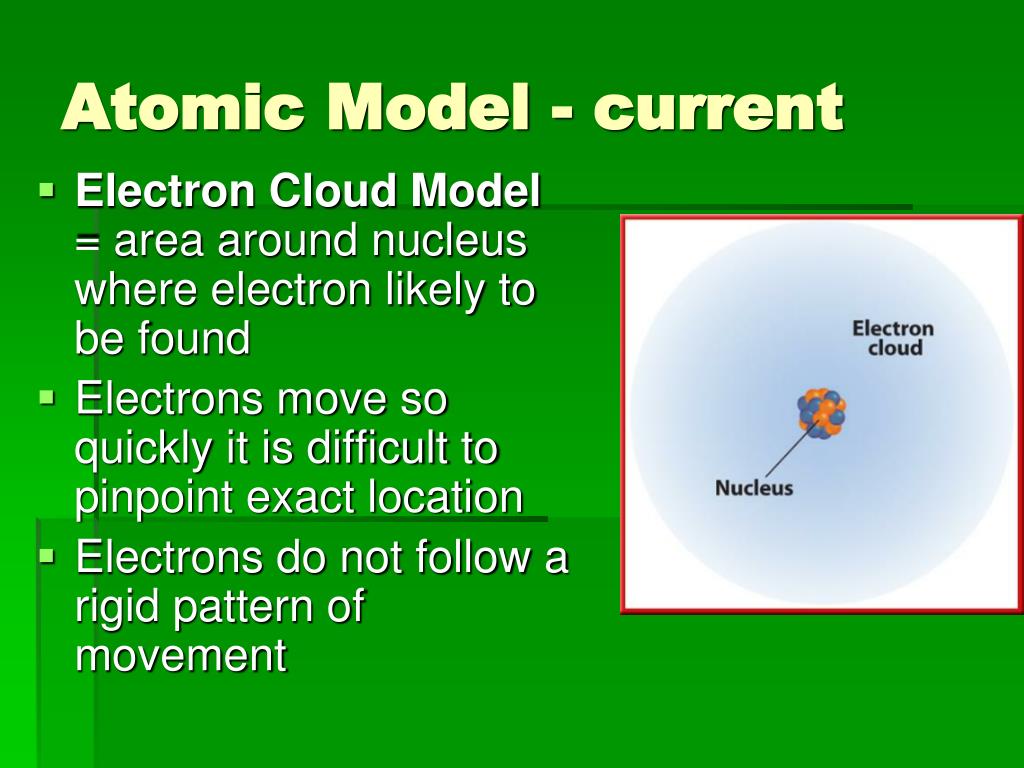
The current model is the Quantum Mechanical Model, also sometimes referred to as the Electron Cloud Model. This model isn't just a snapshot; it's a dynamic, probabilistic description of where electrons are likely to be found within an atom.
A Journey Through Atomic History
Our understanding of the atom has evolved dramatically over time. Early ideas were more philosophical than scientific.
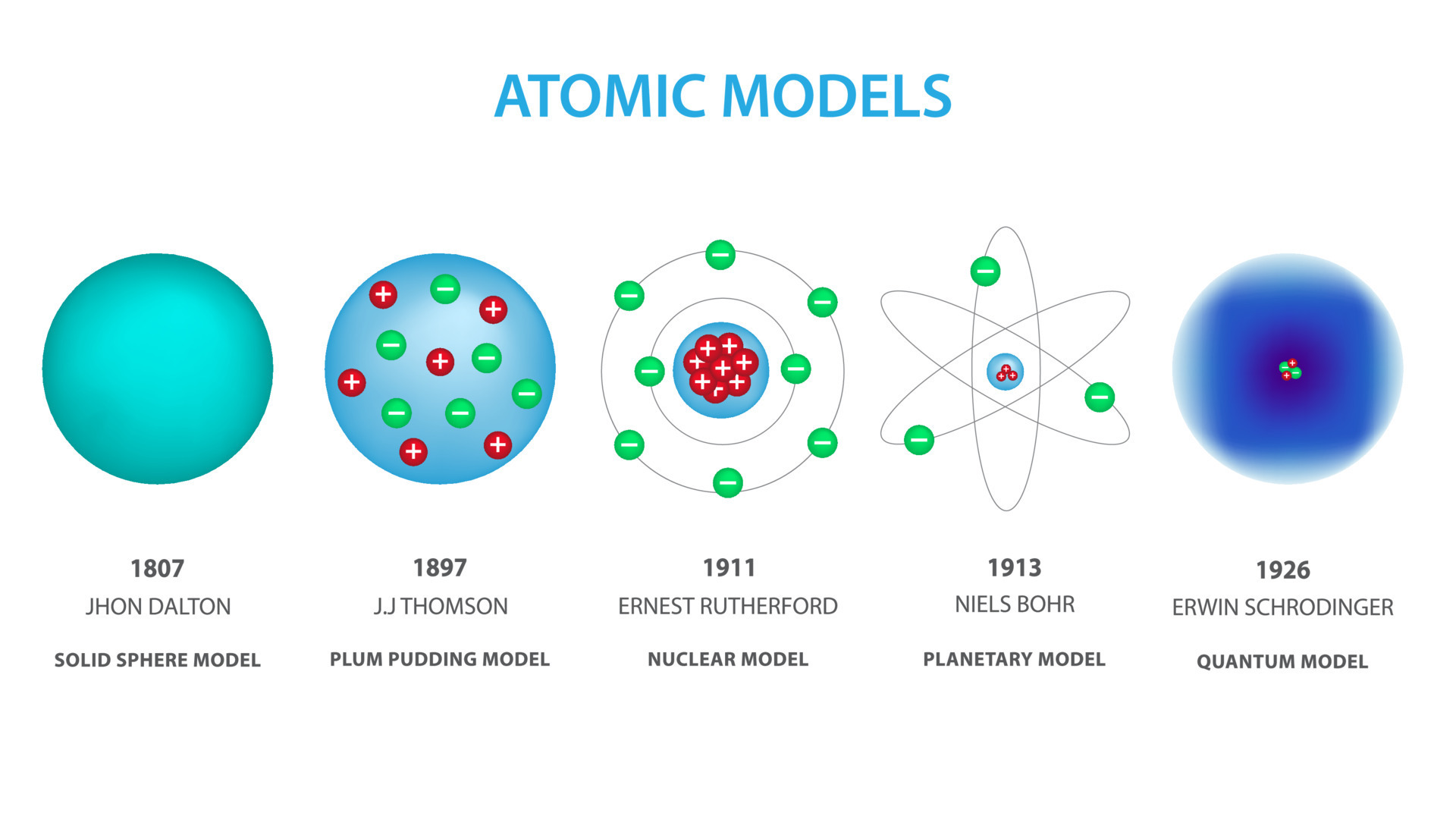
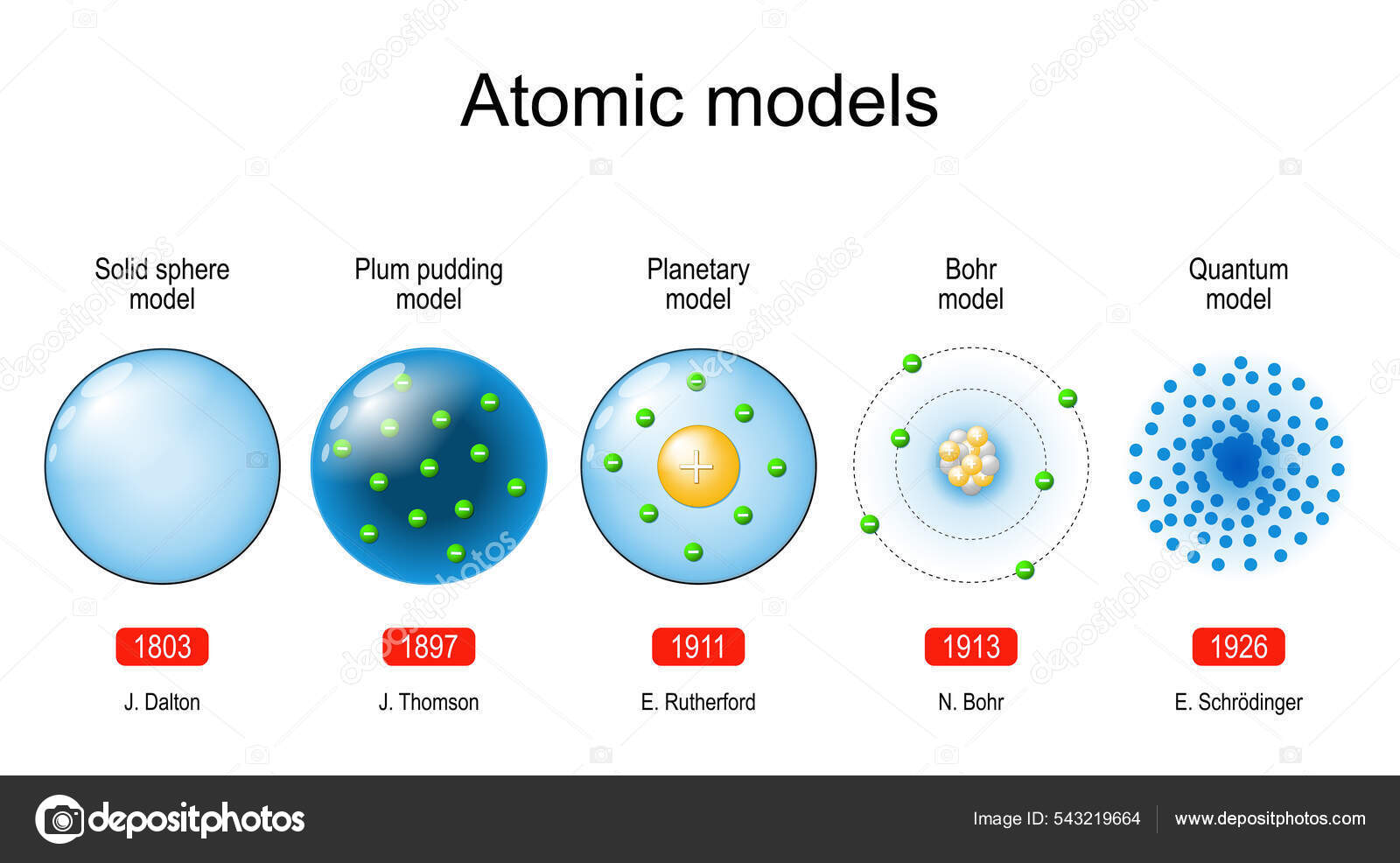
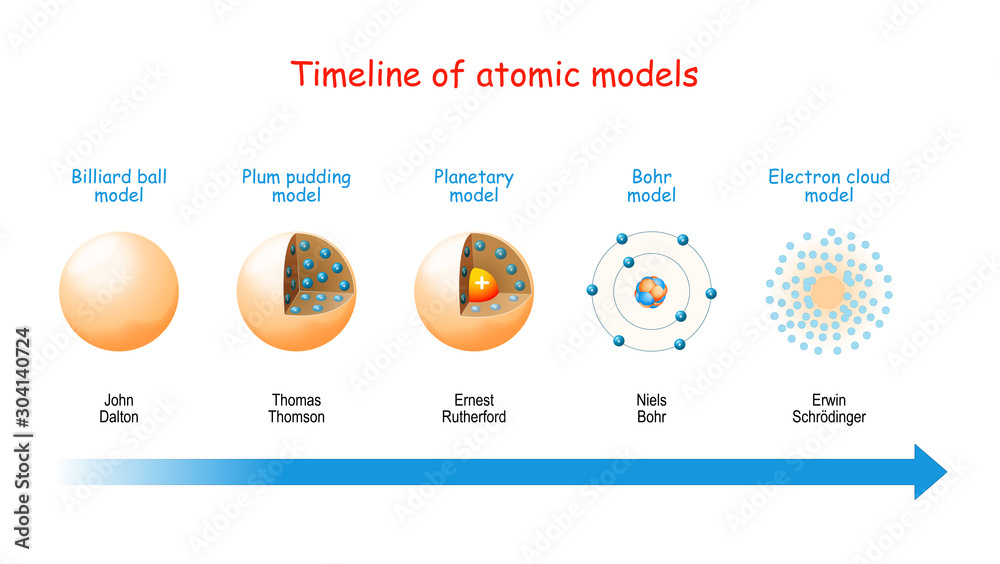
The ancient Greeks, like Democritus, first proposed the idea of indivisible particles, which he named 'atomos.' While insightful, this idea was purely conceptual and lacked any experimental basis.
Dalton's Atomic Theory
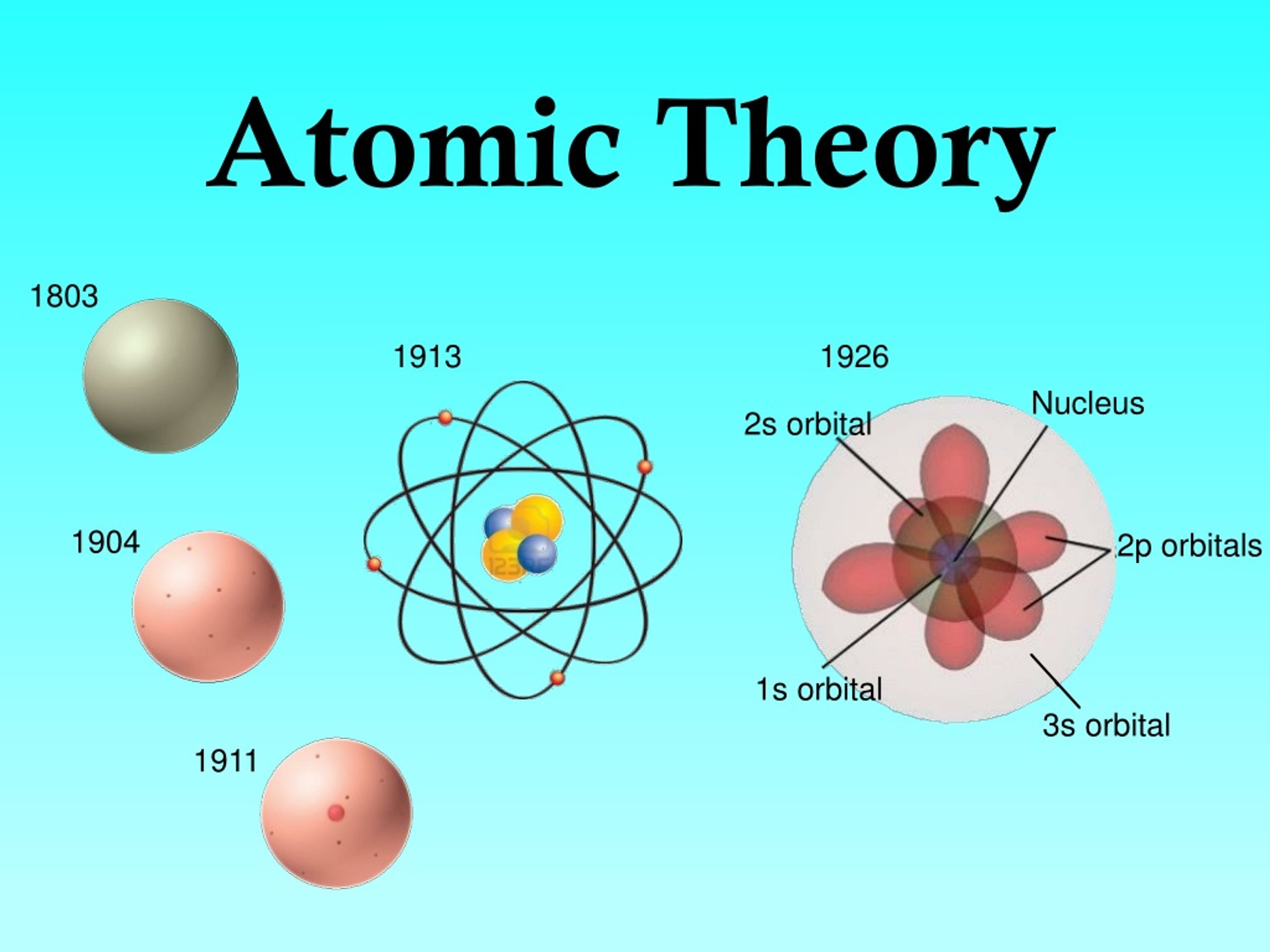
Fast forward to the early 19th century, and we find John Dalton. He revitalized the atomic theory with his own postulates.
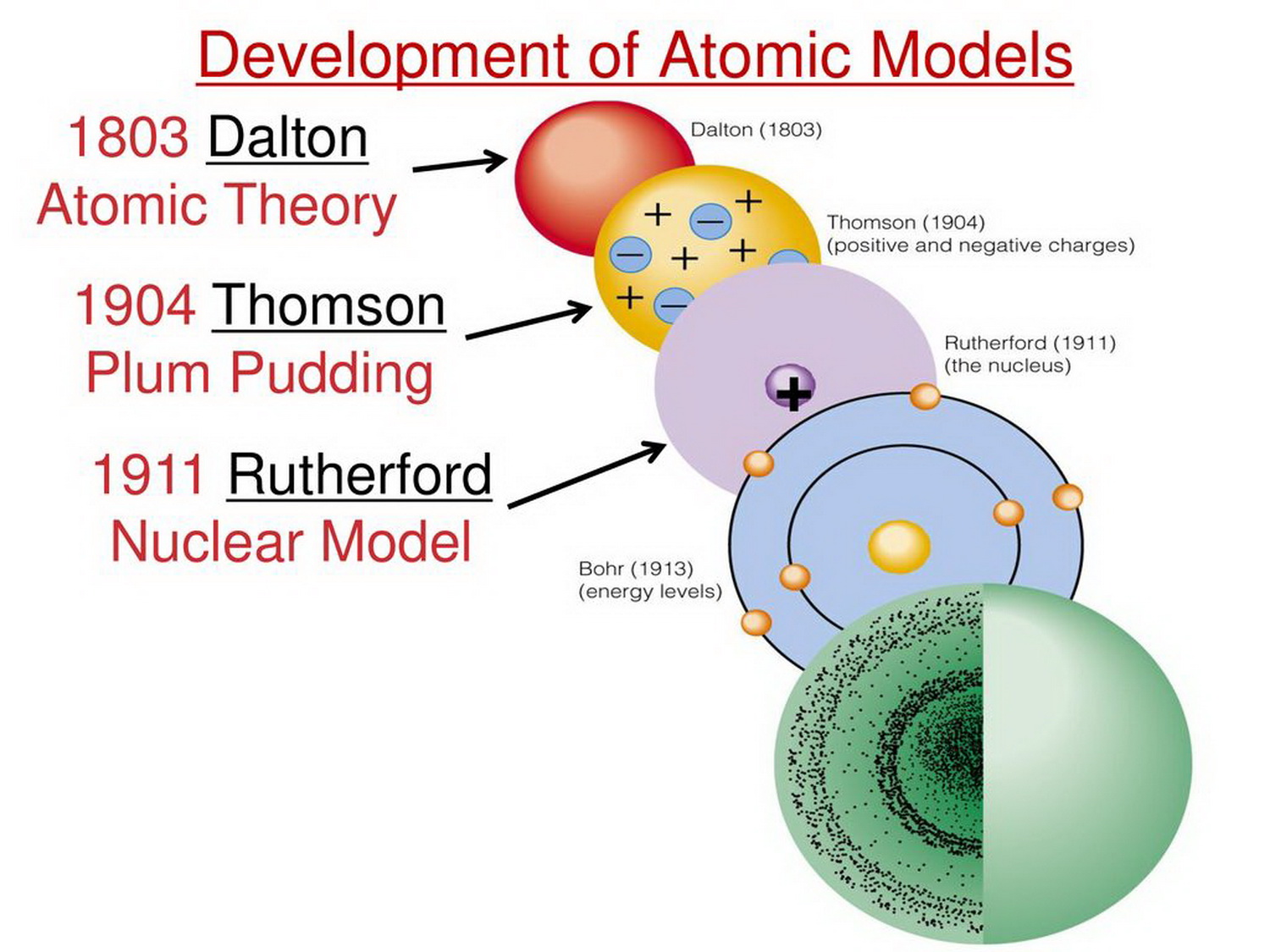
Dalton proposed that all matter is composed of atoms. He also stated that atoms of a given element are identical in mass and properties, and chemical reactions involve the rearrangement of atoms. This laid the foundation for modern chemistry.
Thomson's Plum Pudding Model
The discovery of the electron by J.J. Thomson in 1897 was a major breakthrough. This discovery upended the idea of the atom as an indivisible entity.
Thomson proposed the "plum pudding" model, envisioning the atom as a positively charged sphere with negatively charged electrons scattered throughout, like plums in a pudding.
Rutherford's Nuclear Model
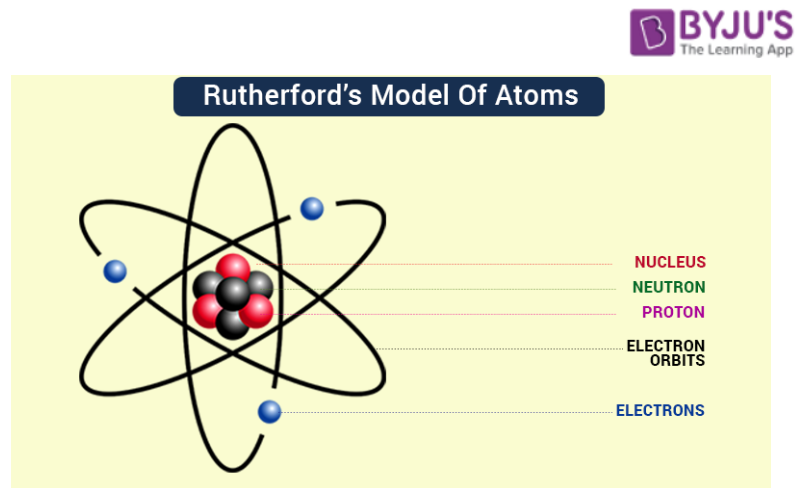
Ernest Rutherford's gold foil experiment in 1911 challenged Thomson's model. Rutherford and his team bombarded a thin gold foil with alpha particles.
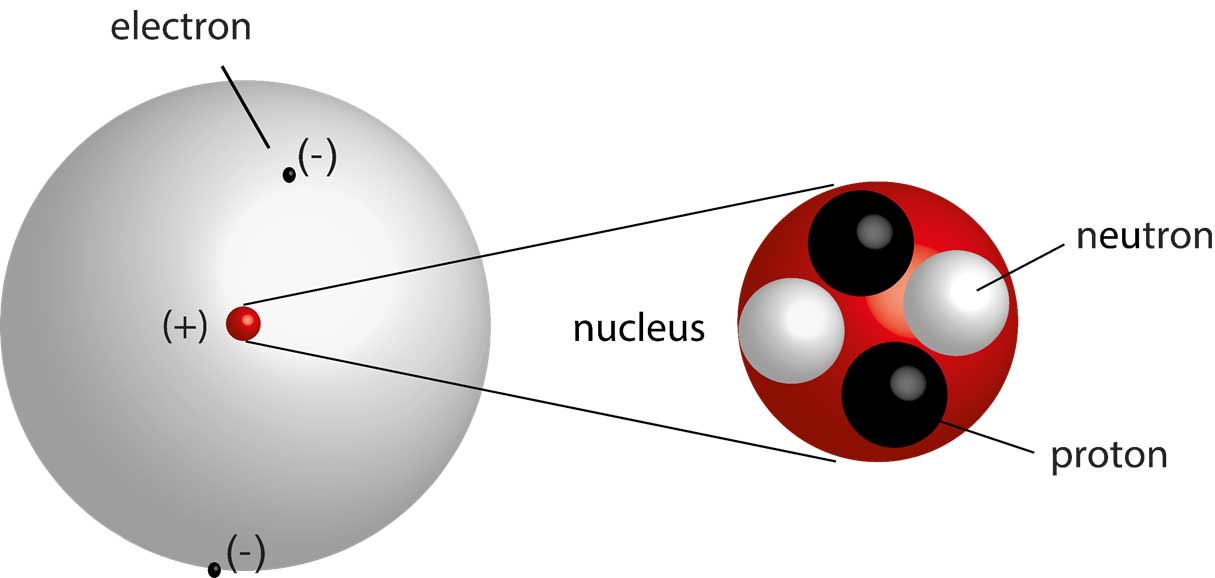
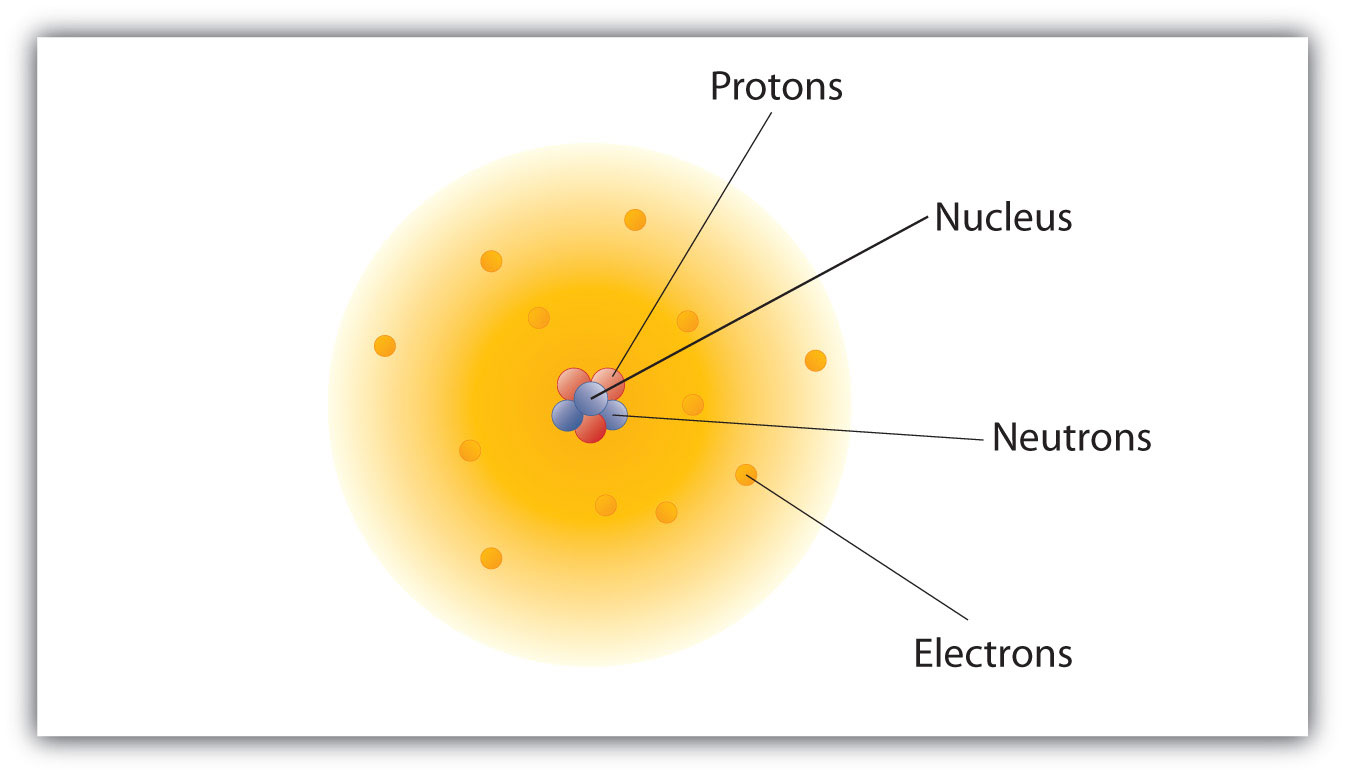
They observed that a small fraction of alpha particles were deflected at large angles, indicating the presence of a small, dense, positively charged nucleus at the center of the atom. This led to the nuclear model, where electrons orbit the nucleus like planets around the sun.
Bohr's Model
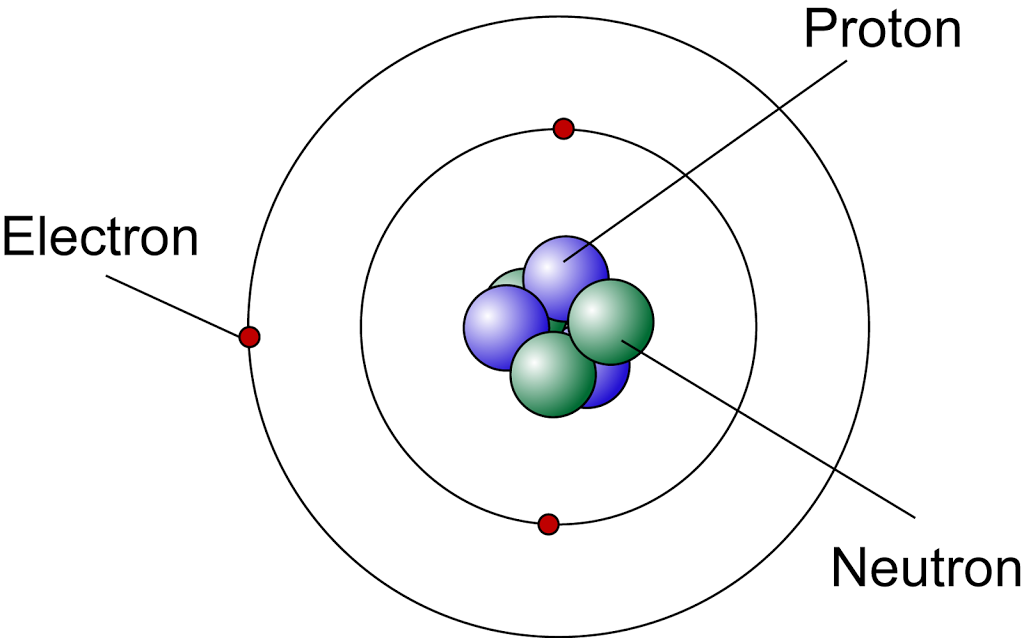
Niels Bohr refined Rutherford's model in 1913 by incorporating quantum theory. He proposed that electrons orbit the nucleus in specific, quantized energy levels or shells.
Electrons could jump between these energy levels by absorbing or emitting energy in the form of photons. Bohr's model successfully explained the discrete line spectra of hydrogen but failed to accurately predict the behavior of more complex atoms.
The Quantum Mechanical Model Takes Center Stage
The limitations of Bohr's model paved the way for the quantum mechanical model. This model, developed in the 1920s, revolutionized our understanding of the atom.
Key figures in its development include Werner Heisenberg, Erwin Schrödinger, and Paul Dirac.
Key Principles of the Quantum Mechanical Model
The Heisenberg Uncertainty Principle states that it's impossible to know both the exact position and momentum of an electron simultaneously. This concept fundamentally challenges the classical notion of electrons following fixed orbits.
Instead, the quantum mechanical model describes electrons in terms of probabilities. It introduces the concept of atomic orbitals, which are regions of space where electrons are most likely to be found.
Schrödinger's equation, a central equation in quantum mechanics, describes the behavior of electrons in atoms. Solutions to this equation yield a set of quantum numbers that characterize the energy and shape of atomic orbitals.
These quantum numbers include the principal quantum number (n), which determines the energy level; the angular momentum quantum number (l), which determines the shape of the orbital; and the magnetic quantum number (ml), which determines the orientation of the orbital in space.
Orbitals, Not Orbits
The quantum mechanical model replaces the concept of fixed orbits with the concept of orbitals. These are three-dimensional regions of space where there is a high probability of finding an electron.
Orbitals have different shapes and energies, described by quantum numbers. The shapes are often represented as s, p, d, and f orbitals.
An s orbital is spherical, while p orbitals are dumbbell-shaped. The d and f orbitals have more complex shapes. These shapes reflect the probabilistic nature of electron location.
Significance of the Quantum Mechanical Model
The quantum mechanical model provides a powerful framework for understanding the behavior of atoms and molecules. It's the foundation for much of modern chemistry and physics.
It allows us to predict and explain chemical bonding. The model also allows us to predict spectral properties, and other phenomena with incredible accuracy.
It explains how atoms interact to form molecules. This understanding is crucial for developing new materials, drugs, and technologies.
The model is used in fields like materials science, drug discovery, and nanotechnology. This model drives innovation across many scientific disciplines.
Beyond the Quantum Mechanical Model
While the quantum mechanical model is incredibly successful, research continues to push the boundaries of our understanding. Scientists are exploring relativistic effects and quantum electrodynamics to refine the model.
These refinements address the behavior of electrons in very heavy atoms. They also address the interactions between electrons and electromagnetic radiation.
These advanced models build upon the foundation of the quantum mechanical model, providing even more accurate descriptions of the atomic world.
Conclusion
The journey to understand the atom has been a long and fascinating one. From the philosophical musings of the ancient Greeks to the sophisticated mathematics of quantum mechanics, each step has brought us closer to unveiling the secrets of matter.
The Quantum Mechanical Model stands as the current pinnacle of our understanding. It emphasizes the probabilistic nature of electrons and their behavior within atoms.
As technology advances and new experiments are conducted, our understanding of the atom will undoubtedly continue to evolve. The quest to understand the fundamental building blocks of the universe is a continuous and exciting endeavor.