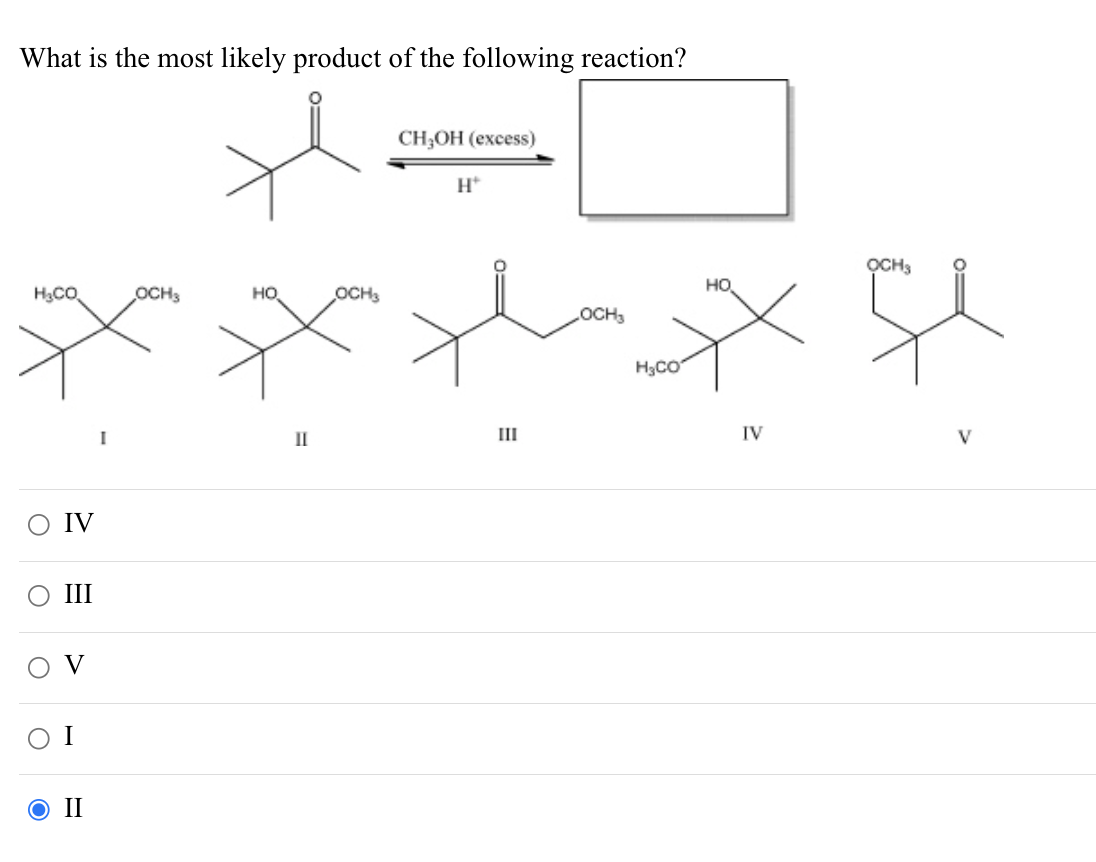
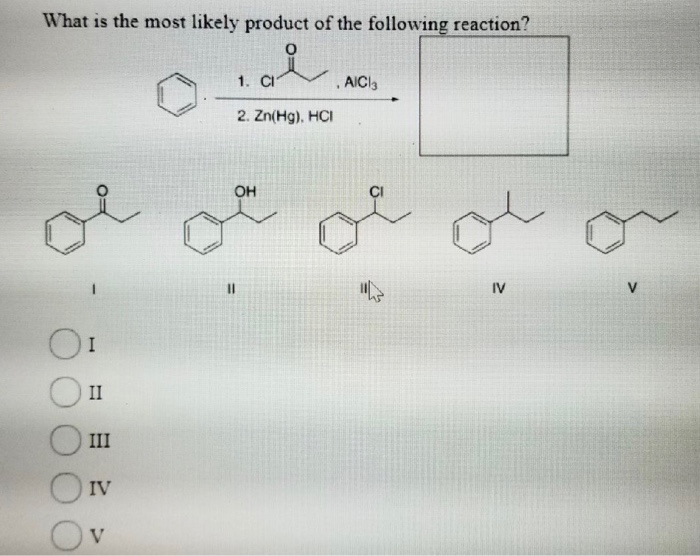
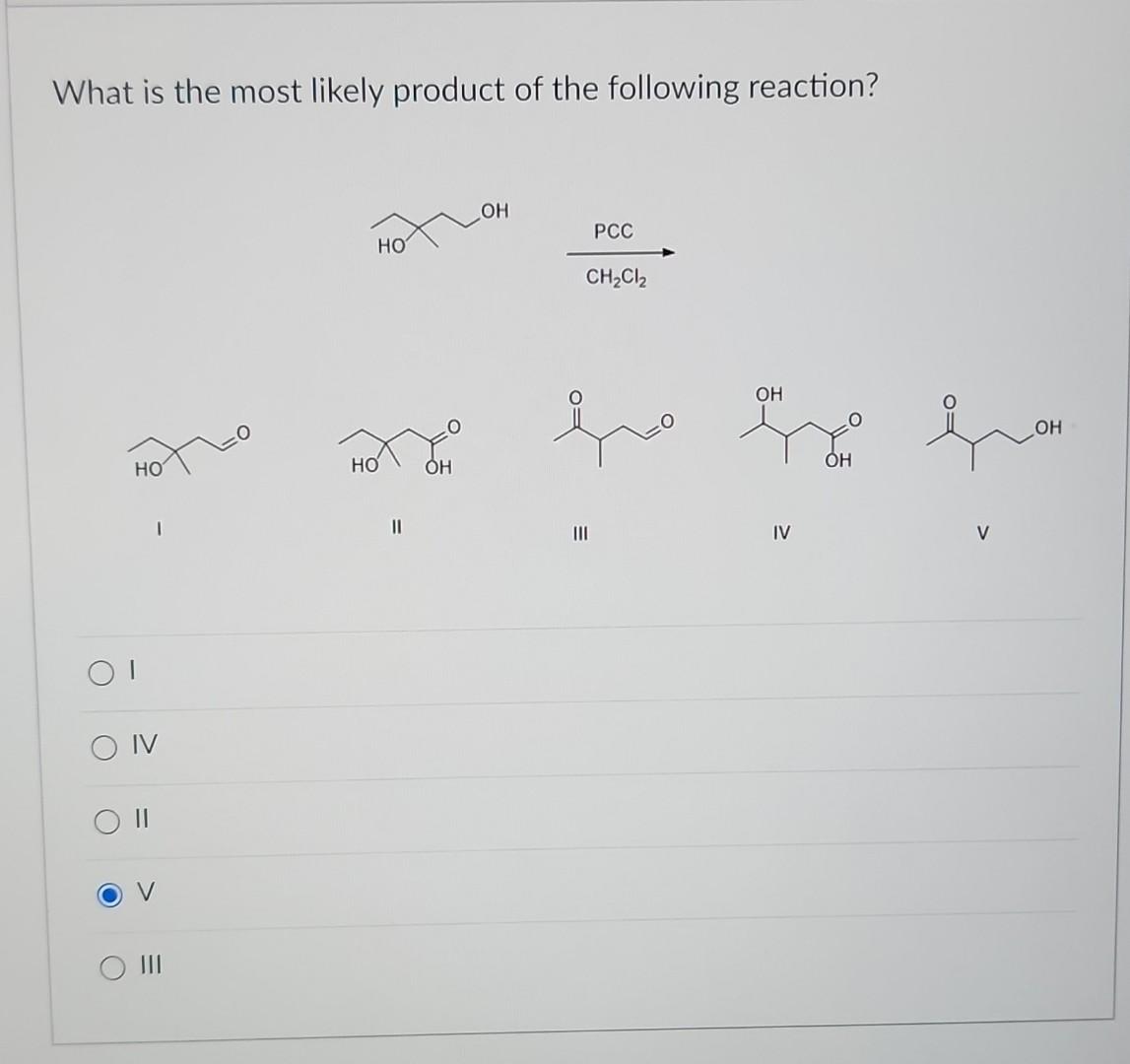
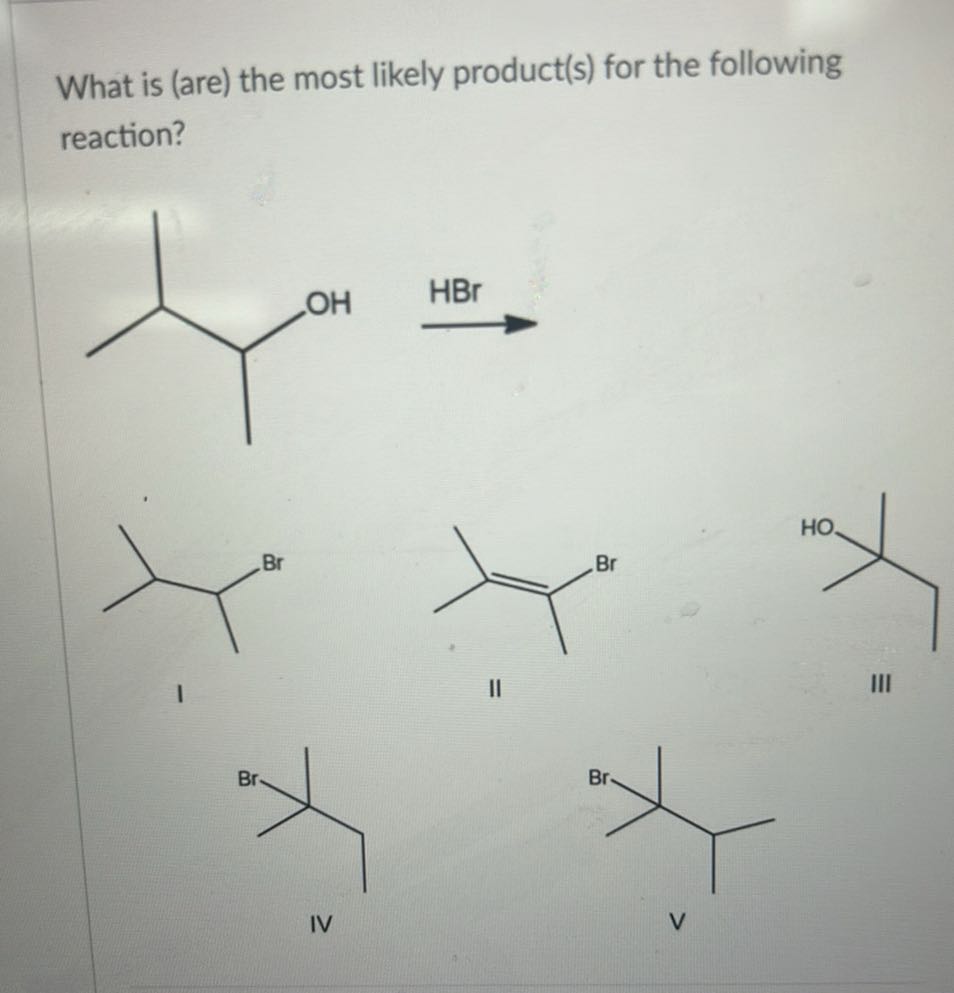
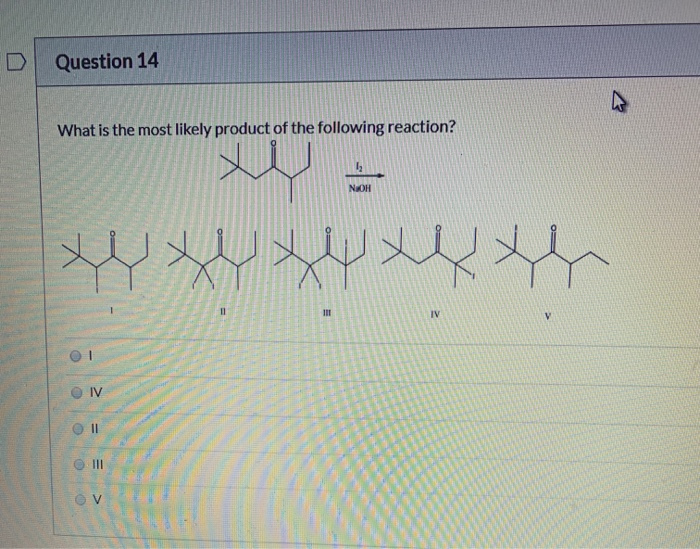
What Is The Most Likely Product For The Following Reaction
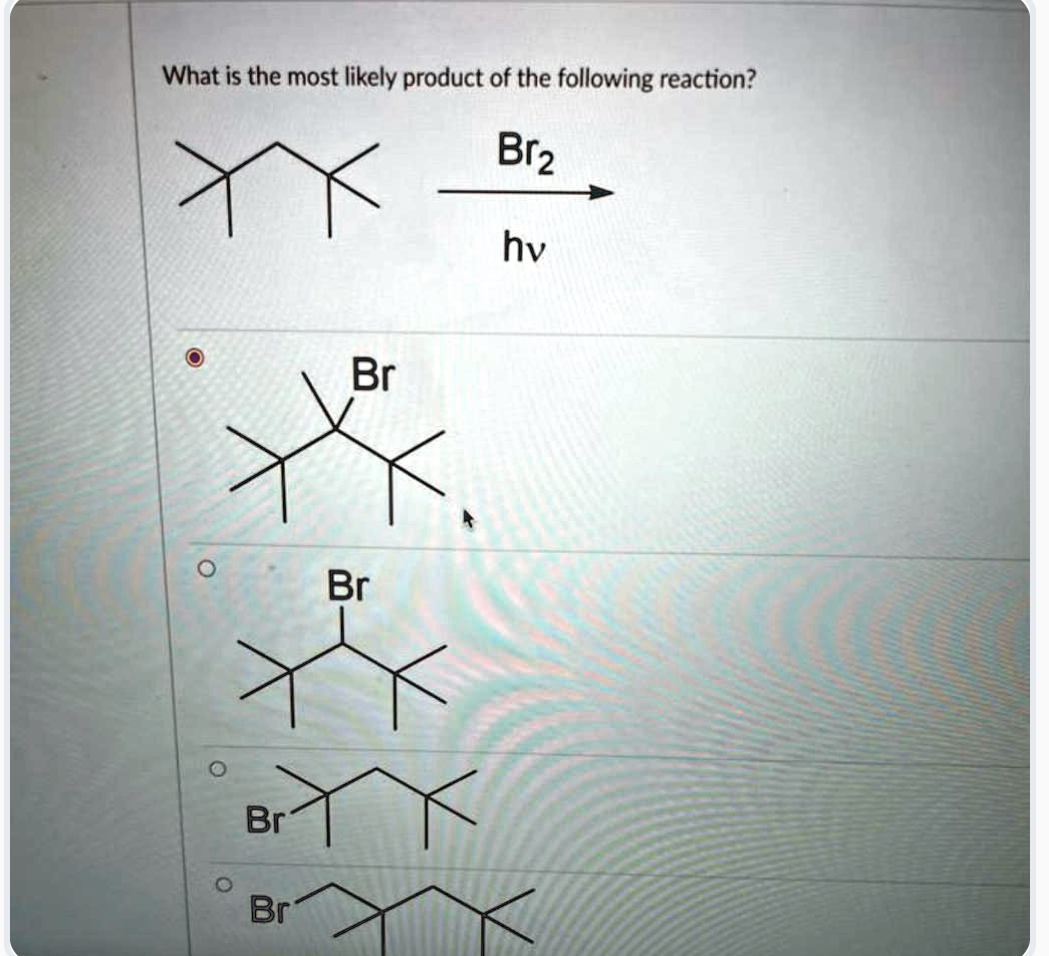
Imagine a chemist in a lab, surrounded by beakers and bubbling solutions. The air crackles with anticipation as they meticulously combine reactants, chasing the elusive goal of synthesizing a desired compound. It's a scene of quiet focus, punctuated by the occasional scribbled note or satisfied nod as the reaction progresses.
The question, "What is the most likely product for the following reaction?" might seem simple, but it opens a gateway into the fascinating world of organic chemistry. Understanding the factors that influence a chemical reaction's outcome is crucial in fields ranging from drug development to materials science.
Let's explore the key concepts involved in predicting the major product of a chemical reaction, offering a window into the chemist's mind as they navigate the intricate pathways of molecular transformations.
Understanding the Basics of Chemical Reactions
At its heart, a chemical reaction involves the rearrangement of atoms and molecules. Reactants interact to form new substances called products, driven by the fundamental principle of minimizing energy.
Chemical reactions are governed by the laws of thermodynamics and kinetics. Thermodynamics tells us whether a reaction is favorable (exothermic, releasing energy) or unfavorable (endothermic, requiring energy). Kinetics tells us how fast a reaction will proceed.
Predicting the major product of a reaction requires a deep understanding of reaction mechanisms. A reaction mechanism is a step-by-step description of how reactants transform into products. Understanding the mechanism allows chemists to predict which products will form preferentially.
Factors Influencing Product Formation
Many factors influence the outcome of a chemical reaction, directing the reaction towards a specific product.
Steric hindrance is one of the most important factors. Bulky groups on a molecule can block the approach of a reactant, favoring reactions at less hindered sites. Imagine trying to thread a needle with boxing gloves on - it's much easier without the gloves.
Electronic effects also play a significant role. Electron-donating groups can stabilize positive charges, while electron-withdrawing groups can stabilize negative charges. These effects influence the reactivity of different sites on a molecule.
The nature of the solvent can have a profound impact on the reaction. Polar solvents favor reactions involving charged intermediates, while nonpolar solvents favor reactions involving neutral intermediates. Think of it like oil and water – they don't mix well, and similarly, reactants and solvents need to be compatible.
Temperature is another critical factor. Higher temperatures generally favor reactions with higher activation energies, leading to different product distributions. Like adding heat to cook food faster, temperature can drastically affect a reaction's speed and outcome.
Specific Reaction Types and Their Predictive Rules
To predict the major product, it is vital to understand the type of reaction being studied.
In addition reactions, two or more molecules combine to form a single molecule. Markovnikov's rule is a famous example of this. In the addition of HX (where X is a halogen) to an alkene, the hydrogen atom will add to the carbon with more hydrogen atoms already attached.
Substitution reactions involve the replacement of one atom or group of atoms with another. SN1 and SN2 reactions are common examples, and their mechanisms dictate the stereochemistry and regiochemistry of the product.
Elimination reactions involve the removal of atoms or groups of atoms to form a multiple bond. Zaitsev's rule states that the major product is the most substituted alkene, the one with the most alkyl groups attached to the double-bonded carbons.
Rearrangement reactions involve the migration of an atom or group of atoms within a molecule. These reactions often occur through carbocation intermediates, which can rearrange to form more stable carbocations. For example, Wagner-Meerwein rearrangements involve alkyl or aryl group shifts.
Tools and Resources for Prediction
Chemists employ a variety of tools and resources to predict the major product of a reaction. These include textbooks, online databases, and computational chemistry software.
Textbooks provide a comprehensive overview of reaction mechanisms and predictive rules. Standard textbooks like Organic Chemistry by Paula Yurkanis Bruice and Organic Chemistry by Kenneth L. Williamson are invaluable resources.
Online databases, such as Reaxys and SciFinder, allow chemists to search for known reactions and their products. These databases contain a wealth of experimental data that can be used to predict the outcome of new reactions.
Computational chemistry software, such as Gaussian and Spartan, can be used to model chemical reactions and predict their products. These tools use quantum mechanical calculations to estimate the energies of reactants, intermediates, and products, providing valuable insights into reaction mechanisms.
Real-World Applications
The ability to predict the major product of a reaction has significant implications in various fields.
In drug discovery, chemists need to synthesize complex molecules with specific biological activities. Predicting the major product of each reaction step is crucial for designing efficient synthetic routes.
In materials science, chemists design and synthesize new materials with desired properties. Understanding reaction mechanisms allows them to control the structure and properties of these materials at the molecular level.
In environmental chemistry, predicting the products of chemical reactions is essential for understanding the fate of pollutants in the environment. This knowledge can be used to develop strategies for remediation and prevention.
The Art and Science of Prediction
Predicting the major product of a chemical reaction is both an art and a science. It requires a deep understanding of chemical principles, combined with intuition and experience.
Experienced chemists often develop a "feel" for how reactions will proceed, based on their knowledge of reaction mechanisms and their experience with similar reactions. This intuition is invaluable for guiding experimental work and interpreting results.
Even with the best tools and knowledge, predicting the major product of a reaction can be challenging. Unexpected results can occur, leading to new discoveries and a deeper understanding of chemical reactivity. Every reaction is a learning experience. The beauty of chemistry lies in its complexity and the constant possibility of surprise.
Ultimately, understanding how to predict the most likely product of a chemical reaction empowers us to design and control the molecular world. This knowledge drives innovation across many disciplines, from medicine to materials science, shaping a future where we can manipulate matter at the most fundamental level.