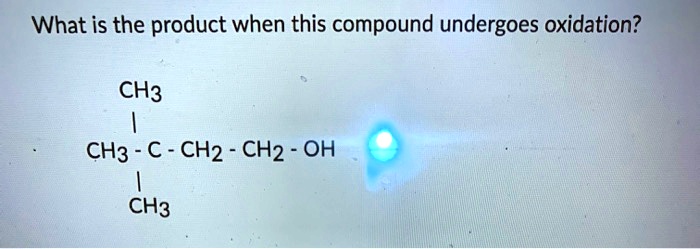
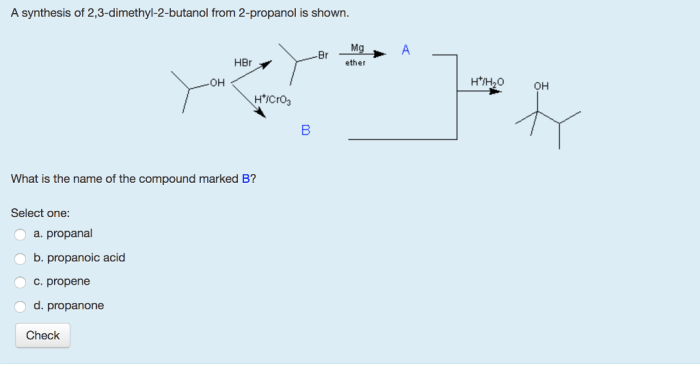
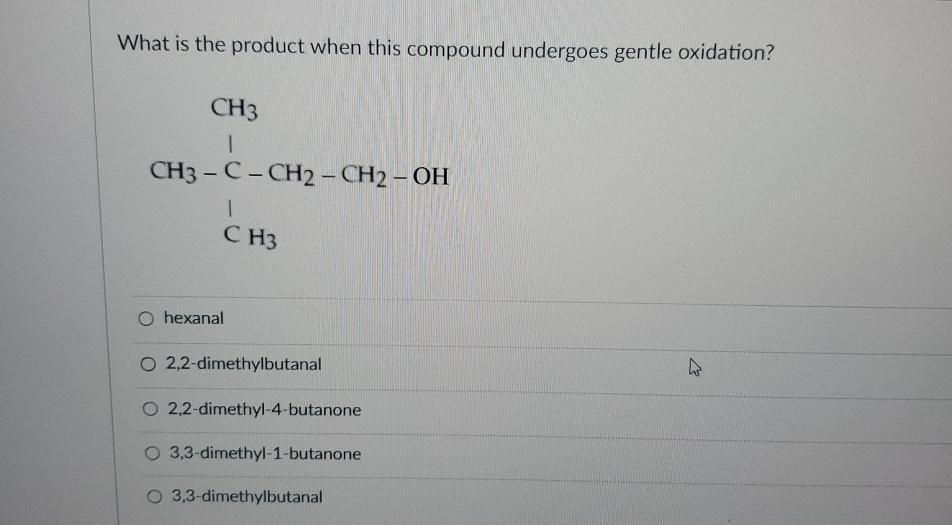
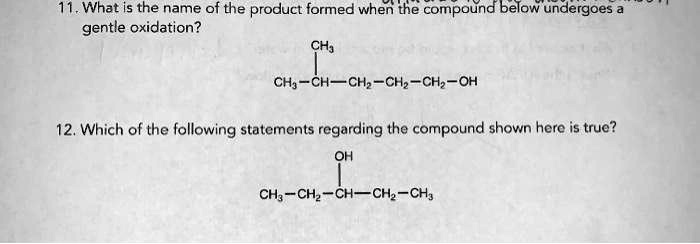
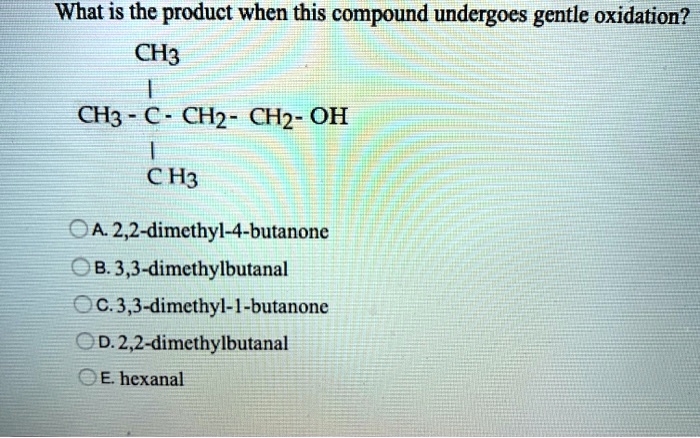
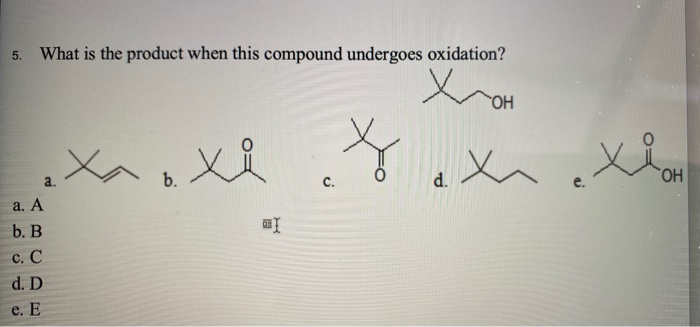
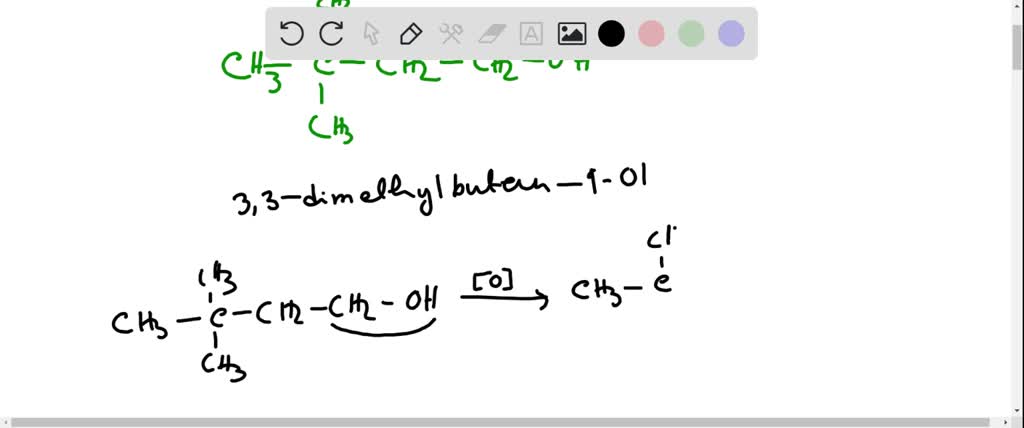
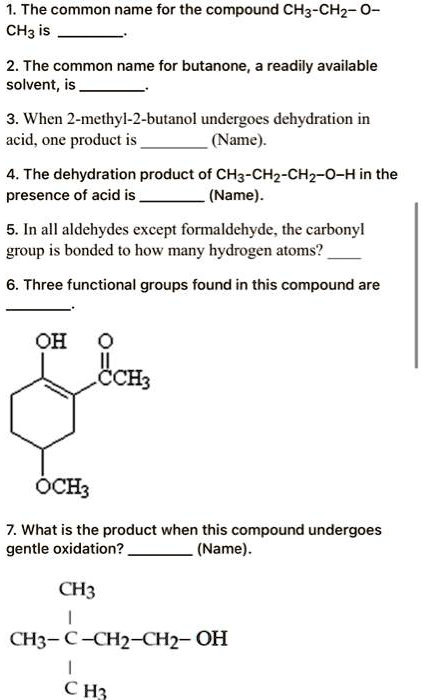
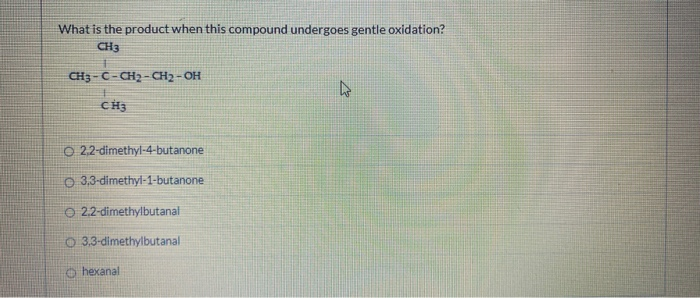
What Is The Product When This Compound Undergoes Gentle Oxidation
Imagine a laboratory, sunlight streaming through the windows, illuminating dust motes dancing in the air. A gentle hum fills the space, the quiet symphony of scientific inquiry. In a glass flask, a clear liquid shimmers, the starting point of our investigation. Today, we're not chasing miracle cures or groundbreaking inventions. Instead, we're embarking on a more subtle journey: unraveling the mystery of what happens when a specific compound undergoes gentle oxidation.
At the heart of this article lies the quest to identify the product formed when benzyl alcohol experiences gentle oxidation. This seemingly simple chemical transformation holds significance for various fields, from organic synthesis to industrial applications. Understanding this reaction allows scientists to predict its behavior, manipulate its properties, and harness its potential for innovation.
The Star of the Show: Benzyl Alcohol
Benzyl alcohol, a colorless liquid with a mild, pleasant odor, is a versatile aromatic alcohol. Its chemical formula is C6H5CH2OH. Its structure features a benzene ring attached to a hydroxylmethyl group, conferring upon it a unique combination of properties.
It is widely used as a solvent, preservative, and fragrance ingredient. You can find it in pharmaceuticals, cosmetics, and flavorings.
Its ubiquity makes understanding its chemical behavior crucial for diverse applications.
Oxidation: A Gentle Touch
Oxidation, in its broadest sense, is the loss of electrons by a molecule, atom, or ion. In organic chemistry, oxidation often manifests as an increase in the number of bonds to oxygen or a decrease in the number of bonds to hydrogen.
Gentle oxidation, in this context, refers to controlled oxidation using mild oxidizing agents under specific conditions. This prevents the reaction from going too far and producing unwanted byproducts.
It's about carefully guiding the reaction to achieve the desired outcome.
Unveiling the Product: Benzaldehyde
When benzyl alcohol undergoes gentle oxidation, the primary product formed is benzaldehyde. This transformation involves the oxidation of the alcohol group (-CH2OH) to an aldehyde group (-CHO).
The reaction can be represented as: C6H5CH2OH → C6H5CHO.
This conversion is a cornerstone reaction in organic chemistry, demonstrating the fundamental principles of oxidation.
The Role of the Oxidizing Agent
The success of this transformation hinges on the selection of the appropriate oxidizing agent. Several reagents can achieve this, each with its own advantages and disadvantages.
Common choices include pyridinium chlorochromate (PCC) and Swern oxidation conditions. These methods provide controlled oxidation, preventing further oxidation to benzoic acid.
The choice of reagent greatly influences the yield and purity of the desired benzaldehyde product.
Why Not Benzoic Acid? The Importance of Control
One might wonder why the oxidation stops at benzaldehyde and doesn't proceed further to benzoic acid (C6H5COOH). The answer lies in the control afforded by gentle oxidation.
Stronger oxidizing agents, or less controlled reaction conditions, would indeed lead to the formation of benzoic acid. However, by using mild reagents and carefully monitoring the reaction, we can selectively produce benzaldehyde in high yields.
Selectivity is key to successful organic synthesis.
The Significance of Benzaldehyde
Benzaldehyde is far more than just a chemical intermediate. It is a valuable compound in its own right, with a wide array of applications across various industries.
Perhaps its most well-known application is as the primary component of artificial almond flavoring. Its characteristic aroma contributes to the distinctive taste of marzipan, amaretto, and other almond-flavored treats.
Beyond its culinary uses, benzaldehyde serves as a precursor for the synthesis of numerous other organic compounds. These compounds find use in pharmaceuticals, dyes, and agrochemicals.
Its versatility makes it an indispensable building block in modern chemistry.
Industrial Applications and Beyond
The controlled oxidation of benzyl alcohol to benzaldehyde is not just a laboratory curiosity. It is a process employed on an industrial scale to meet the global demand for benzaldehyde.
Improved catalytic methods are under constant development for the efficient production of benzaldehyde. These advancements aim to reduce waste, lower energy consumption, and increase the overall sustainability of the process.
Ongoing research seeks to discover novel applications for benzaldehyde and its derivatives.
A Deeper Dive into the Mechanism
The specific mechanism of the oxidation reaction depends on the oxidizing agent employed. Let's consider the example of PCC oxidation.
PCC, a complex of chromium trioxide with pyridine and hydrochloric acid, reacts with benzyl alcohol to form a chromate ester. This ester then undergoes a series of steps involving proton transfer and elimination to yield benzaldehyde.
Understanding the mechanism allows chemists to optimize reaction conditions, improve yields, and minimize unwanted side reactions.
Environmental Considerations
While the oxidation of benzyl alcohol to benzaldehyde is a valuable reaction, it's important to consider its environmental impact.
Traditional oxidizing agents, such as chromium-based reagents, can generate hazardous waste. Therefore, researchers are actively exploring more environmentally friendly alternatives, such as catalytic oxidation using air or oxygen.
The pursuit of sustainable chemistry is driving innovation in this field.
Looking Ahead
The gentle oxidation of benzyl alcohol serves as a microcosm of the broader field of organic chemistry. It illustrates the power of controlled reactions to transform molecules and create valuable compounds.
Future research will likely focus on developing more efficient, sustainable, and selective methods for this transformation. This includes exploring novel catalysts, alternative oxidizing agents, and innovative reaction conditions.
The journey of discovery continues, driven by curiosity and a commitment to innovation.
Reflecting on this process, from the initial vision in the flask to the multitude of applications of benzaldehyde, highlights the beauty and power of chemical transformations. It's a reminder that even seemingly simple reactions can have profound implications for our world. The gentle oxidation of benzyl alcohol is not just a chemical equation; it's a story of discovery, innovation, and the enduring quest to understand the intricate workings of the molecular world.