What Temperature Does Salt Stop Melting Ice
The biting wind whipped across the frozen lake, turning exposed skin crimson. Skaters, bundled in layers, glided across the crystalline surface, their laughter echoing in the crisp air. But near the shoreline, a different scene unfolded: someone diligently scattering salt, the small white crystals disappearing into the ice, a silent battle against the relentless freeze.
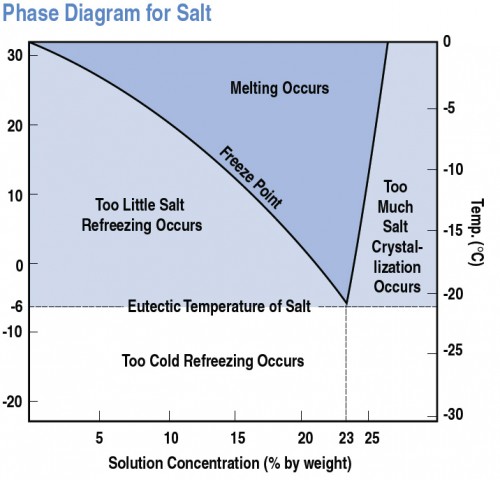
The question of how effective salt is at melting ice is a common one. While salt is a familiar winter tool, its power isn't limitless; it stops working at a certain temperature. Understanding this threshold, typically around 15°F (-9°C), is crucial for safe winter navigation and effective ice management.
The Science of Salt and Ice
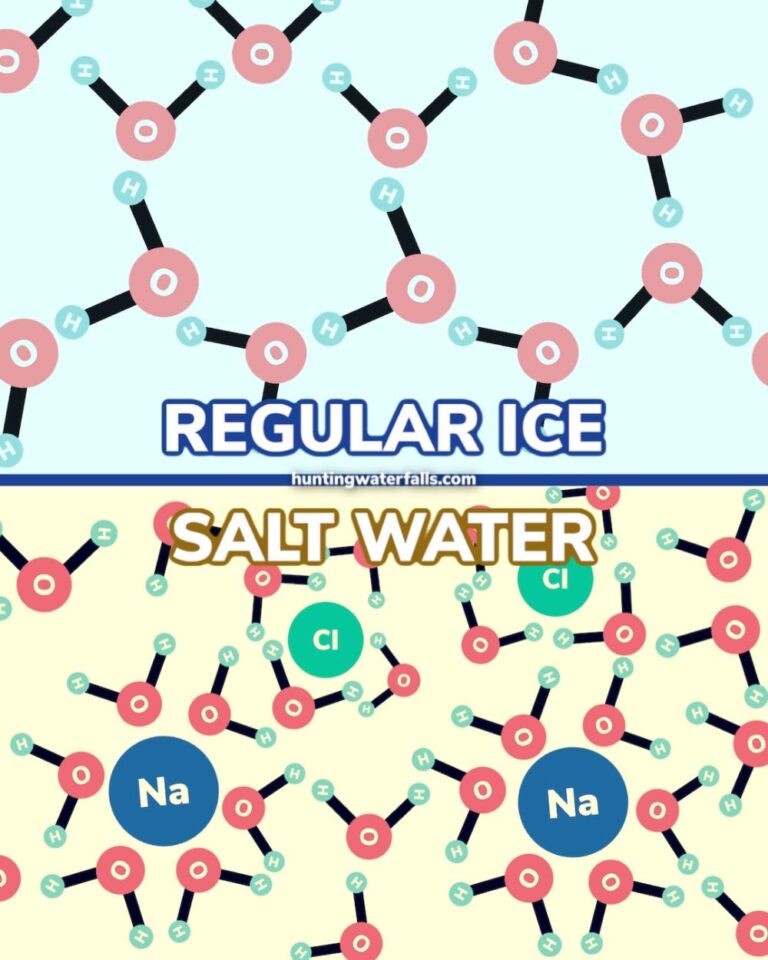
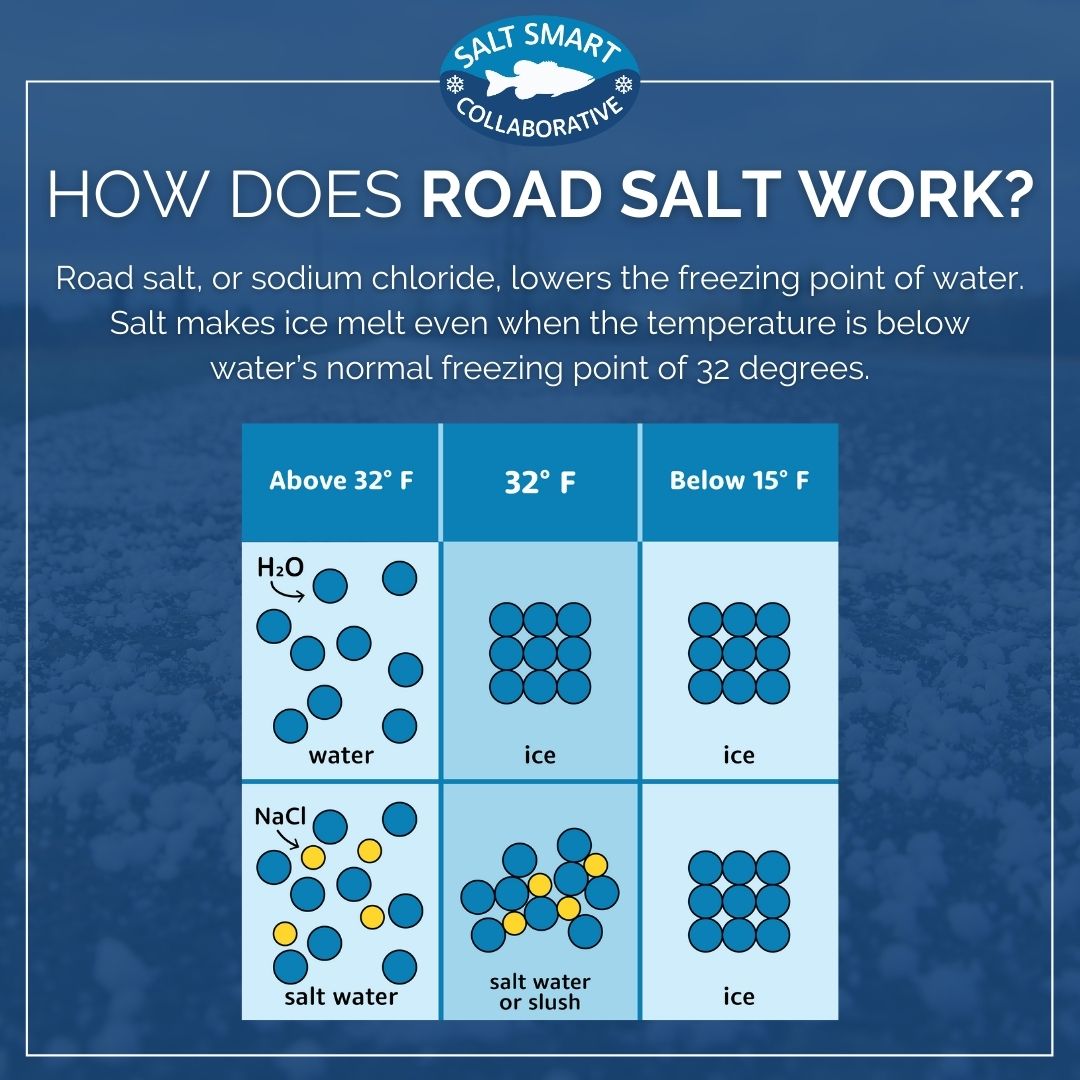
To understand why salt stops melting ice at a certain temperature, we need to delve into the science of freezing point depression. Pure water freezes at 32°F (0°C). Salt, or sodium chloride (NaCl), interferes with water molecules' ability to form the organized crystalline structure of ice.
When salt dissolves in water, it separates into sodium (Na+) and chloride (Cl-) ions. These ions disrupt the formation of hydrogen bonds between water molecules, lowering the freezing point of the water.
Think of it as the salt molecules getting in the way. They block the water molecules from forming their nice, orderly ice lattice.
The Temperature Threshold: Why 15°F?
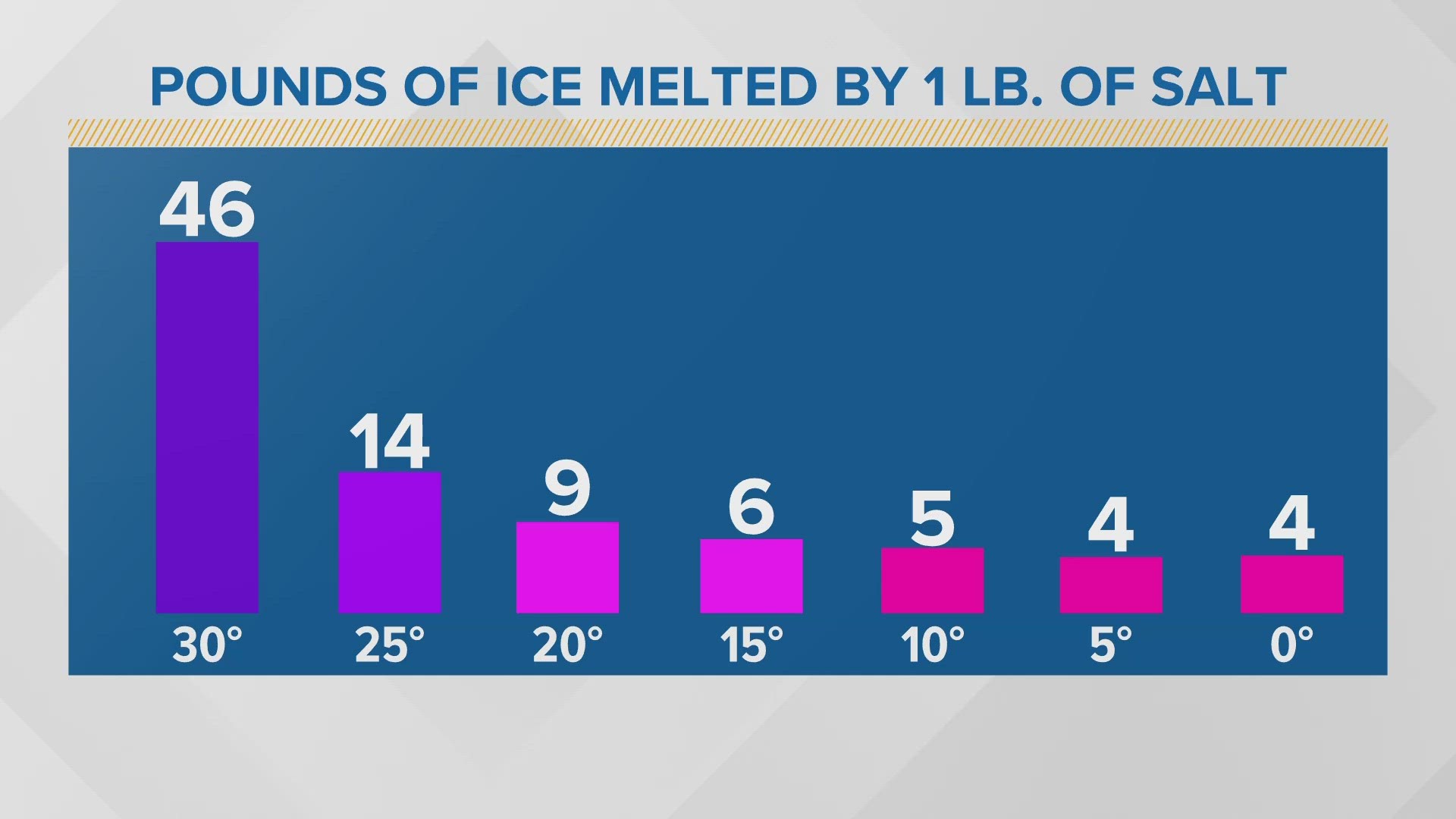
The effectiveness of salt as a de-icer decreases as the temperature drops. At temperatures significantly below 15°F (-9°C), the concentration of salt needed to lower the freezing point further becomes impractically high.
Essentially, the water is already so close to its freezing point that the salt can't overcome the strong intermolecular forces enough to keep the water from freezing. You would need to use an extreme amount of salt for a small amount of melting.
Furthermore, the rate at which salt dissolves in water slows down dramatically at lower temperatures. The process of dissolving and interfering with the ice formation becomes too sluggish to be effective.
Beyond Salt: Other De-Icing Options
When temperatures plummet below the point where salt is effective, other de-icing alternatives come into play. These options generally work by lowering the freezing point of water to a greater degree than salt.
Calcium chloride (CaCl2) is a common alternative. It can be effective down to approximately -25°F (-32°C). It's stronger than NaCl.
Magnesium chloride (MgCl2) is another option, often considered less corrosive than calcium chloride. However, its effectiveness also diminishes at very low temperatures, but can still be used down to -13°F (-25°C).
Sand and gravel don't melt ice, but instead they provide traction. These materials are valuable for improving grip on icy surfaces, particularly when de-icing chemicals aren't effective or environmentally desirable.
Environmental Considerations
The use of de-icing salts and chemicals can have environmental consequences. Excessive salt runoff can contaminate waterways, harming aquatic life and affecting drinking water sources. Over salinization is a very real threat to bodies of water.
It can also damage vegetation along roadsides and contribute to the corrosion of vehicles and infrastructure. It is imperative to practice responsible salting practices.
Many communities are adopting more sustainable strategies for winter road maintenance. These include using less salt, employing brine solutions (salt dissolved in water) before storms to prevent ice from bonding to the pavement, and utilizing alternative de-icers that are less harmful to the environment.
Practical Applications and Safe Winter Practices
Understanding the temperature limitations of salt is essential for ensuring safe winter travel. Motorists should be aware that icy conditions can persist even after roads have been treated with salt, particularly during extremely cold weather.
Adjusting driving habits to account for reduced traction is critical. Slow down, increase following distances, and avoid sudden braking or steering maneuvers.
Homeowners should also be mindful of the temperature when applying salt to driveways and walkways. Using excessive amounts of salt when it's too cold to be effective is not only wasteful but also potentially harmful to the environment and nearby plants.
A Look at the Numbers
Data from the National Weather Service provides valuable insights into local temperature trends. Checking the forecast before heading out is crucial for preparing for winter weather conditions.
Many state and local transportation departments also provide real-time road condition information, including reports on ice and snow cover. They also publish snow plow routes that you can use.
These resources can help individuals and communities make informed decisions about winter travel and safety precautions.
The Ongoing Search for Better Solutions
Scientists and engineers are continually researching new and improved de-icing methods. This pursuit involves exploring alternative chemicals, developing more efficient application techniques, and investigating innovative materials that can prevent ice formation in the first place.
Some researchers are exploring the use of plant-based de-icers, such as sugar beet juice, as a more environmentally friendly alternative to traditional salts. These organic compounds can lower the freezing point of water while also providing nutrients to roadside vegetation.
The goal is to find solutions that are both effective and sustainable, minimizing the environmental impact of winter road maintenance while ensuring public safety.
Conclusion
The simple act of scattering salt on ice hides a complex interaction of science and environmental impact. While salt remains a valuable tool in our winter arsenal, recognizing its temperature limitations and embracing more sustainable practices is essential.
As the icy winds of winter continue to blow, a deeper understanding of the delicate balance between safety, science, and environmental responsibility will guide us toward a more resilient and sustainable future. By continuing to be conscientious and informed, we can better navigate the icy challenges winter throws our way.
Ultimately, the most effective solution comes down to education and responsible usage. Use the least amount of salt required and be aware of the temperature!